Oscillating Pattern in Nanoparticle Crystallisation
Max Planck researchers in Potsdam demonstrate an oscillating pattern in nanoparticle crystallisation and self-organisation
Advertisement
In order to survive, biological systems need to form patterns and organise themselves. Scientists at the Max Planck Institute for colloids and Interfaces in Potsdam, Germany, have now combined self-organisation with chemical pattern formation.
Scientists are especially interested in oscillating chemical reactions. These occur when reaction products periodically and repeatedly change. Their behaviour is of importance to many fields of study - including chaos research. That is because these reaction systems are always complex and far away from thermodynamic equilibrium. One particularly well-known example is the "Belousov-Zhabotinsky" reaction.
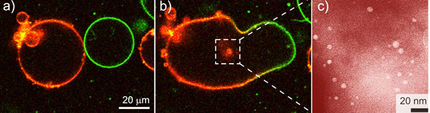
Mathematically, spatially oscillating reactions can be described as "reaction-diffusion systems". This means that it is not just chemical reactions which influence the amount of material at a certain point in space. Diffusion also plays a role - the exchange of material with the surrounding area. In such simulations, we get the typical concentric circle pattern of a Belousov-Zhabotinsky reaction. In the picture above, it is indicated in red-violet.
Researchers from Potsdam have now proven that these oscillating reactions can also apply to multi-phase systems, and even to the self-organisation processes of nanoparticles. What is central is that in a multi-phase reaction system, it is possible to formulate either an autocatalyic or autoinhibiting reaction step. This leads an oscillating system to be constructed, and ultimately a pattern to be formed.
The researchers used a newly synthesized polymer to create the typical concentric circle pattern, via controlled barium carbonate crystallisation. Such patterns correspond quite well to the calculations in a simulation. The researchers also were able to formulate a complex coupled reaction system including crystallisation, complexation, and precipitation reactions and identify the autocatalytic formation of a complex between barium and the polymer.
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.