Molecular Solomon's Knot
Self-organization leads to intertwined molecular rings
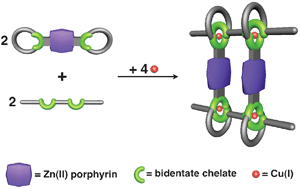
It has been a symbol for centuries - and is now a molecule: Solomon's knot, a motif consisting of two doubly intertwined rings. A team of researchers from the University of California, Los Angeles (USA), and Nottingham Trent University (UK) have now used a self-organization process to get molecular building blocks to weave themselves into a Solomon-type knot. "The secret of our success is the careful selection of metal ions and solvents," revealed J. Fraser Stoddart in the journal Angewandte Chemie. "Although various molecular species compete with each other in solution, the Solomon's knot wins out during the crystallization process simply because it crystallizes better." Systems consisting of individual molecular components that are not chemically bound to each other, but rather are tied together through purely mechanical means, are an enormous challenge for scientists. Stoddart, one of the pioneers in the area of supramolecular chemistry, has successfully produced a whole series of such structures. For example, he and his team have produced a system of molecules in the form of Borromean rings, whose name is derived from an Italian family that used such interlocked rings in their crest. Stoddart's Borromean rings are formed from an 18-component self-assembly process in which six organic pieces with two "teeth" and another six with three "teeth" grip six zinc ions, producing the mutually interlocked three ring system. Things get particularly interesting when zinc and copper ions are mixed in a 1:1 ratio: a 12-component self-assembly process ensues to interlock two rings twice over instead of three, resulting in the formation of a molecular Solomon knot, isolated upon crystallization. The four loops of the knot are stabilized by two copper and two zinc ions. In solution, there is initially an equilibrium between the different types of knots. During crystallization, the Solomon's knot form is preferred over the Borromean rings. Original publication: J. Fraser Stoddart et al.; "A Molecular Solomon Link"; Angewandte Chemie International Edition 2007, 46, No. 1, 218-222.
Most read news
Topics
Organizations
Other news from the department science
These products might interest you

ERBAdry by CARLO ERBA Reagents
Anhydrous solvents from CARLO ERBA Reagents in a clever redesign
ERBAdry series impresses with the latest generation of septa and sealing caps

Thermo Scientific™ Dionex™ ASE™ 150 or 350 Accelerated Solvent Extractor systems by Thermo Fisher Scientific
Accelerated Solvent Extraction (ASE) – Maximize results and reduce errors in food analysis!
More extractions in less time using less solvent

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.