Carbon rising from the flames
Advertisement
Scientists in the US offer new insights into how polycyclic aromatic pollutants are formed in flames and other combustion processes.
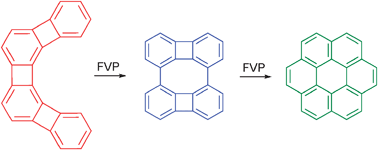
Peter Vollhardt and colleagues from the University of California, and William Karney from the University of San Francisco, in the US, use flash-vacuum-pyrolytic reorganisation of angular [4]phenylene to obtain new data on the mechanisms by which polycyclic aromatic pollutants are formed in combustion processes.
Significantly, Vollhardt and his team provide data on a new form of carbon using this process. Unlike graphite, which consists of fused (six-membered) benzene rings; this new structure is composed of fused alternating 4-, 6- and 8-membered rings. Such a carbon allotrope is calculated to be electronically highly activated and therefore has potential as a novel semiconductor.
"The challenge is to modify the method to improve yields, perhaps through the discovery of catalysts," says Vollhardt. In the future, he hopes to extend the study to synthesise larger phenylene sheet substructures in order to access other electronic and photonic materials.
Original article: Dosa et. al.; "Flash-vacuum-pyrolytic reorganization of angular [4]phenylene"; Chem. Commun. 2009
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
































































