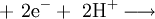To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electrolysis
In chemistry and manufacturing, electrolysis is a method of separating chemically bonded elements and compounds by passing an electric current through them. Additional recommended knowledge
OverviewElectrolysis involves the passage of an electric current through, in general, an ionic substance that is either molten or dissolved in an aqueous solution resulting in chemical reactions at the electrodes. The positive electrode is called the anode, and the negative is the cathode. An ionic compound is dissolved with an appropriate solvent, or melted by heat, so that its ions are available in the liquid. An electrical current is applied between a pair of inert electrodes immersed in the liquid. Each electrode attracts ions that are of the opposite charge. Therefore, positively-charged ions (called cations) move towards the cathode, whereas negatively-charged ions (termed anions) move toward the anode. The energy required to separate the ions, and cause them to gather at the respective electrodes, is provided by an electrical power supply. At the probes, electrons are absorbed or released by the ions, forming a collection of the desired element or compound. Oxidation of anions can take place at the anode, and the reduction of cations at the cathode. For example, it is possible to oxidize cations at the anode:
It is also possible to reduce anions at the cathode:
Neutral molecules can also react at either electrode. For example: In electrolysis, the anode is the positive electrode, meaning it has a deficit of electrons; species in contact with the anode can be stripped of electrons (i.e., they are oxidized). The cathode is the negative electrode, meaning it has a surplus of electrons. Species in contact with the cathode tend to gain electrons (i.e., they are reduced). The amount of electrical energy that must be added equals the change in Gibbs free energy of the reaction plus the losses in the system. The losses can (in theory) be arbitrarily close to zero, so the maximum thermodynamic efficiency equals the enthalpy change divided by the free energy change of the reaction. In most cases, the electric input is larger than the enthalpy change of the reaction, so some energy is released in the form of heat. In some cases, for instance, in the electrolysis of steam into hydrogen and oxygen at high temperature, the opposite is true. Heat is absorbed from the surroundings, and the heating value of the produced hydrogen is higher than the electric input. (It is worth noting that the maximum theoretic efficiency of a fuel cell is the inverse of that of electrolysis. It is, thus, impossible to create a perpetual motion machine by combining the two processes. See water fuel cell for an example of such an attempt.) A higher current flow (amperage) through the cell means it will be passing more electrons through it at any given time. This means a faster rate of reduction at the cathode and a faster rate of oxidation at the anode. This corresponds to a greater number of moles of product. The amount of current that passes depends on the conductance of the electrodes and electrolyte, though it also depends on how much current the power source itself can generate. Current also makes a difference in that it can shift chemical equilibria by sheer mass action. The processes in an electrolytic cell with just two or three reactants can become very complex. Most of the time it is best to search the literature to see what current density works best for a desired process. For instance, metals plated at a certain current density might form a durable and shiny coating on the substrate, whereas some other current density might form an excessively grainy, dull coating. A higher potential difference (voltage) applied to the cell means the cathode will have more energy to bring about reduction, and the anode will have more energy to bring about oxidation. Higher potential difference enables the electrolytic cell to oxidize and reduce energetically more "difficult" compounds. This can drastically change what products will form in a given experiment. On a practical level, both current and voltage determine what will form in a cell. The following technologies are related to electrolysis:
Electrolysis of water
One important use of electrolysis of water is to produce hydrogen.
This has been suggested as a way of shifting society toward using hydrogen as an energy carrier for powering electric motors and internal combustion engines. (See hydrogen economy.) Electrolysis of water can be observed by passing direct current from a battery or other DC power supply through a cup of water (in practice a saltwater solution increases the reaction intensity making it easier to observe). Using platinum electrodes, hydrogen gas will be seen to bubble up at the cathode, and oxygen will bubble at the anode. If other metals are used as the anode, there is a chance that the oxygen will react with the anode instead of being released as a gas. For example, using iron electrodes in a sodium chloride solution electrolyte, iron oxide will be produced at the anode, which will react to form iron hydroxide. When producing large quantities of hydrogen, this can significantly contaminate the electrolytic cell - which is why iron is not used for commercial electrolysis. The energy efficiency of water electrolysis varies widely. The efficiency is a measure of what fraction of electrical energy used is actually contained within the hydrogen. Some of the electrical energy is converted to heat, a useless by-product. Some reports quote efficiencies between 50% and 70%[1] This efficiency is based on the Lower Heating Value of Hydrogen. The Lower Heating Value of Hydrogen is thermal energy released when Hydrogen is combusted. This does not represent the total amount of energy within the Hydrogen, hence the efficiency is lower than a more strict definition. Other reports quote the theoretical maximum efficiency of electrolysis. The theoretical maximum efficiency is between 80% and 94%.[2]. The theoretical maximum considers the total amount of energy absorbed by both the hydrogen and oxygen. These values refer only to the efficiency of converting electrical energy into hydrogen's chemical energy. The energy lost in generating the electricity is not included. For instance, when considering a power plant that converts the heat of nuclear reactions into hydrogen via electrolysis, the total efficiency is more like 25%–40%.[3] About four percent of hydrogen gas produced worldwide is created by electrolysis, and normally used onsite. Hydrogen is used for the creation of ammonia for fertilizer via the Haber process, and converting heavy petroleum sources to lighter fractions via hydrocracking. There is some speculation about future development of hydrogen as an energy carrier. ExperimentersScientific pioneers of electrolysis included:
More recently, electrolysis of heavy water was performed by Fleischmann and Pons in their famous experiment, allegedly resulting in anomalous heat generation and the controversial claim of cold fusion. Faraday's laws of electrolysisSee Also: Faraday's laws of electrolysis First law of electrolysisIn 1832, Michael Faraday reported that the quantity of elements separated by passing an electrical current through a molten or dissolved salt is proportional to the quantity of electric charge passed through the circuit. This became the basis of the first law of electrolysis.m = k * q Second law of electrolysisFaraday also discovered that the mass of the resulting separated elements is directly proportional to the atomic masses of the elements when an appropriate integral divisor is applied. This provided strong evidence that discrete particles of matter exist as parts of the atoms of elements. Industrial uses
Electrolysis has many other uses:
See also
Categories: Electrochemistry | Chemical processes | Electrolysis | Hydrogen production |
|||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electrolysis". A list of authors is available in Wikipedia. | |||||||||||





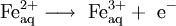 .
.
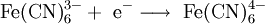 .
.