To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Elementary reactionAn elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state.[1] Product highlightIn a unimolecular elementary reaction a molecule, A, dissociates or isomerises to form the products(s). The rate of such a reaction, at constant temperature, is proportional to the concentration of the species A In a bimolecular elementary reaction, two atoms, molecules, ions or radicals, A and B, react together to form the product(s) The rate of such a reaction, at constant temperature, is proportional to the product of the concentrations of the species A and B. This rate expression can be derived from first principles by using collision theory. The rate expression for an elementary bimolecular reaction is sometimes referred to as the Law of Mass Action as it was first proposed by Guldberg and Waage in 1864. An example of this type of reaction is a cycloaddition reaction. Three chemical species must react simultaneously with each other in a trimolecular elementary reactions. It follows that such reactions are very rare. Any chemical reaction can be broken down into a set of elementary reactions. It is not always possible to derive an overall rate equation for non-trivial reaction schemes, but analytical solutions are possible in favourable cases, see, for example, the steady state approximation or Michaelis-Menten kinetics for enzyme-based reactions. Notes
Categories: Chemical kinetics | Physical chemistry |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Elementary_reaction". A list of authors is available in Wikipedia. |





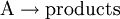
![\frac{d[\mbox{A}]}{dt}=-k[\mbox{A}]](images/math/e/b/0/eb0c763eafe9740fbf56c911643d0b79.png)
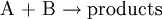
![\frac{d[\mbox{A}]}{dt}=\frac{d[\mbox{B}]}{dt}=-k[\mbox{A}][\mbox{B}]](images/math/4/0/8/408c1898b3446219ddfb8be9ed99d361.png)


