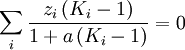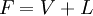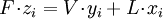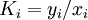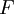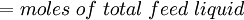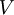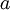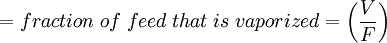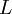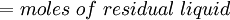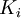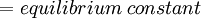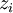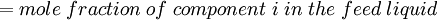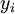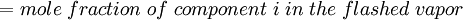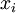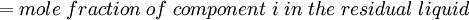To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Flash evaporationFlash (or partial) evaporation is the partial vaporization that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve or other throttling device. This process is one of the simplest unit operations. If the throttling valve or device is located at the entry into a pressure vessel so that the flash evaporation occurs within the vessel, then the vessel is often referred to as a flash drum. If the saturated liquid is a single-component liquid (for example, liquid propane or liquid ammonia), a part of the liquid immediately "flashes" into vapor. Both the vapor and the residual liquid are cooled to the saturation temperature of the liquid at the reduced pressure. This is often referred to as "auto-refrigeration" and is the basis of most conventional vapor compression refrigeration systems. If the saturated liquid is a multi-component liquid (for example, a mixture of propane, isobutane and normal butane), the flashed vapor is richer in the more volatile components than is the remaining liquid. Product highlight
Flash evaporation of a single-component liquidThe flash evaporation of a single-component liquid is an isenthalpic (i.e., constant enthalpy) process and is often referred to as an adiabatic flash. The following equation, derived from a simple heat balance around the throttling valve or device, is used to predict how much of a single-component liquid is vaporized.
If the enthalpy data required for the above equation is unavailable, then the following equation may be used.
( Note: The words "upstream" and "downstream" refer to before and after the liquid passes through the throttling valve or device.) This type of flash evaporation is used in the desalination of brackish water or ocean water by "Multi-Stage Flash Distillation." The water is heated and then routed into a reduced-pressure flash evaporation "stage" where some of the water flashes into steam. This steam is subsequently condensed into salt-free water. The residual salty liquid from that first stage is introduced into a second flash evaporation stage at a pressure lower than the first stage pressure. More water is flashed into steam which is also subsequently condensed into more salt-free water. This sequential use of multiple flash evaporation stages is continued until the design objectives of the system are met. A large part of the world's installed desalination capacity uses multi-stage flash distillation. Typically such plants have 24 or more sequential stages of flash evaporation. Equilibrium flash of a multi-component liquidThe equilibrium flash of a multi-component liquid may be visualized as a simple distillation process using a single equilibrium stage. It is very different and more complex than the flash evaporation of single-component liquid. For a multi-component liquid, calculating the amounts of flashed vapor and residual liquid in equilibrium with each other at a given temperature and pressure requires a trial-and-error iterative solution. Such a calculation is commonly referred to as an equilibrium flash calculation. It involves solving the following Rachford Rice equation:[1][2][3][4][5]
Newton's method (also known as the Newton-Raphson method) is an efficient iterative algorithm for solving the above Rachford Rice equation. Alternatively, an Excel spread sheet and the Excel Solver function can be used. The equilibrium flash of multi-component liquids is very widely utilized in petroleum refineries, petrochemical and chemical plants and natural gas processing plants. (See mole fraction for a definition of that terminology). Spray dryingSpray drying is the rapid drying of a slurry of very small solids suspended in a liquid. The slurry is first atomized into very small liquid droplets which are then sprayed into a stream of hot dry air. The liquid rapidly evaporates leaving behind dry powder or dry solid granules. The dry powder or solid granules are recovered from the exhaust air by using cyclones, bag filters or electrostatic precipitators. A brief explanation of spray drying has been included here because some readers may consider spray drying to be a form of flash evaporation. However, although it is a form of liquid evaporation, it is quite different from flash evaporation. See alsoReferences
Categories: Chemical engineering | Chemical processes | Fluid dynamics | Thermodynamics | Unit operations |
|||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Flash_evaporation". A list of authors is available in Wikipedia. |