To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Introduction to entropy
Thermodynamic entropy provides a measure of certain aspects of energy in relation to absolute temperature. In thermodynamics, entropy is one of the three basic thermodynamic potentials: U (internal energy), S (entropy) and A (Helmholtz energy). Entropy is a measure of the uniformity of the distribution of energy. Product highlight
ExplanationThe concept of thermodynamic entropy is central to the second law of thermodynamics, which deals with physical processes and whether they occur spontaneously. In a general sense the second law says that temperature differences between systems in contact with each other tend to even out and that work can be obtained from these non-equilibrium differences, but that loss of heat occurs, in the form of entropy, when work is done. The concept of energy is central to the first law of thermodynamics, which deals with the conservation of energy and under which the loss in heat will result in a decrease in the internal energy of the thermodynamic system. Thermodynamic entropy provides a comparative measure of the amount of this decrease in internal energy of the system and the corresponding increase in internal energy of the surroundings at a given temperature. A simple and more concrete visualisation of the second law is that energy of all types changes from being localized to becoming dispersed or spread out, if it is not hindered from doing so. Entropy change is the quantitative measure of that kind of a spontaneous process: how much energy has flowed or how widely it has become spread out at a specific temperature. Entropy has been developed to describe any of several phenomena, depending on the field and the context in which it is being used. Information entropy takes the mathematical concepts of statistical thermodynamics into areas of probability theory unconnected with heat and energy. Entropy is an integral part of the second law of thermodynamics, which can be stated as saying that: Temperature differences between thermodynamic systems in contact with each other tend to even out and that work can be obtained from these non-equilibrium differences, but that loss of heat occurs, in the form of entropy, when work is done In this form it provides a measure of the extent to which a heat engine can never completely recycle unused heat into work as a perpetual motion machine might, but will always convert some of the heat into internal energy due to intermolecular interactions, which is not available to do work. Entropy also relates to all kinds of energy and to other fields of science such as chemistry, where the entropy of materials measures the energy required to raise the material to its state at a given temperature from absolute zero. In calculations, entropy is symbolised by S and is a measure at a particular instant, a state function. Thus entropy as energy Q in relation to absolute temperature T is expressed as S = Q/T. Often change in entropy, symbolised by ΔS, is referred to in relation to change in energy, δQ. Statistical mechanics introduces calculation of entropy using probability theory to find the number of possible microstates at an instant, any one of which will contain all the energy of the system at that instant. The calculation shows the probability, which is enabled by the energy: in terms of heat, by the motional energy of molecules. Statistical mechanical entropy is mathematically similar to Shannon entropy which is part of information theory, where energy is not involved. This similarity means that some probabilistic aspects of thermodynamics are replicated in information theory.
Example of entropy increasingIce melting provides a classic example in which entropy increases in a small 'universe', a thermodynamic system consisting of the 'surroundings' (the warm room) and the 'system' of glass, ice, cold water which has been allowed to reach thermodynamic equilibrium at the melting temperature of ice. In this universe, some heat energy δQ from the warmer room surroundings at 298 K (77°F, 25°C) will spread out to the cooler system of ice and water at its constant temperature T of 273 K (32°F, 0°C), the melting temperature of ice. Thus, the entropy of the system, which is δQ/T, increases by δQ/273 K. (The heat δQ for this process is the energy required to change water from the solid state to the liquid state, and is called the enthalpy of fusion, i.e. the ΔH for ice fusion.) It is important to realize that the entropy of the surrounding room decreases less than the entropy of the ice and water increases: the room temperature of 298 K is larger than 273 K and therefore the ratio, (entropy change), of δQ/298 K for the surroundings is smaller than the ratio (entropy change), of δQ/273 K for the ice+water system. This is always true in spontaneous events in a thermodynamic system and it shows the predictive importance of entropy: the final net entropy after such an event is always greater than was the initial entropy. As the temperature of the cool water rises to that of the room and the room further cools imperceptibly, the sum of the δQ/T over the continuous range, “at many increments”, in the initially cool to finally warm water can be found by calculus. The entire miniature ‘universe’, i.e. this thermodynamic system, has increased in entropy. Energy has spontaneously become more dispersed and spread out in that ‘universe’ than when the glass of ice + water was introduced and became a 'system' within it. Origins and usesOriginally, entropy was named to describe the "waste heat", or more accurately energy losses, from heat engines and other mechanical devices which could never run with 100% efficiency in converting energy into work. Later, the term came to acquire several additional descriptions as more came to be understood about the behavior of molecules on the microscopic level. In the late 19th century the word "disorder" was used by Ludwig Boltzmann in developing statistical views of entropy using probability theory to describe the increased molecular movement on the microscopic level. That was before quantum behavior came to be better understood by Werner Heisenberg and those who followed. Descriptions of thermodynamic (heat) entropy on the microscopic level are found in statistical thermodynamics and statistical mechanics. For most of the 20th century textbooks tended to describe entropy as "disorder", following Boltzmann's early conceptualisation of the motional energy of molecules. More recently there has been a trend in chemistry and physics textbooks to describe entropy in terms of "dispersal of energy". Entropy can also involve the dispersal of particles, which are themselves energetic. Thus there are instances where both particles and energy disperse at different rates when substances are mixed together. The mathematics developed in statistical thermodynamics were found to be applicable in other disciplines. In particular, information sciences developed the concept of information entropy where a constant replaces the Temperature which is inherent in thermodynamic entropy. Heat and entropyAt a microscopic level kinetic energy of molecules is responsible for the temperature of a substance or a system. “Heat” is the kinetic energy of molecules being transferred: when motional energy is transferred from hotter surroundings to a cooler system, faster moving molecules in the surroundings collide with the walls of the system and some of their energy gets to the molecules of the system and makes them move faster.
The important overall principle is that ”Energy of all types changes from being localized to becoming dispersed or spread out, if it is not hindered from doing so. Entropy (or better, entropy change) is the quantitative measure of that kind of a spontaneous process: how much energy has been transferred/T or how widely it has become spread out at a specific temperature. Classical calculation of entropyWhen entropy was first defined and used in 1865 the very existence of atoms was still controversial and there was no concept that temperature was due to the motional energy of molecules or that “heat” was actually the transferring of that motional molecular energy from one place to another. Entropy change, ΔS, was described in macro terms that could be measured, such as volume or temperature or pressure. The 1865 equation, which is still completely valid, is that
Introductory descriptions of entropyTraditionally, 20th century textbooks have introduced entropy as order and disorder so that it provides "a measurement of the disorder or randomness of a system". Ambiguities in the terms used (such as "disorder" and "chaos") contribute to widespread confusion and can hinder comprehension of entropy for most students. A more recent formulation describing Entropy as energy dispersal describes entropy as measuring "the spontaneous dispersal of energy — at a specific temperature." Notes and references |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Introduction_to_entropy". A list of authors is available in Wikipedia. |





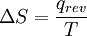 . This can be expanded, part by part, in modern terms of how molecules are responsible for what is happening. Here is that equation expanded:
. This can be expanded, part by part, in modern terms of how molecules are responsible for what is happening. Here is that equation expanded:
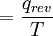 .
.
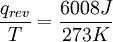 or
or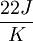 .
.
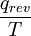 ) from 300 K to 310 K, measure the amount of energy transferred at dozens or hundreds of temperature increments, say from 300.00 K to 300.01 K and then 300.01 to 300.02 and so on, dividing the q by each T, and finally adding them all.
) from 300 K to 310 K, measure the amount of energy transferred at dozens or hundreds of temperature increments, say from 300.00 K to 300.01 K and then 300.01 to 300.02 and so on, dividing the q by each T, and finally adding them all.
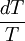 from
from 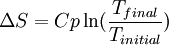 .
.


