To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Statistical thermodynamicsIn thermodynamics, statistical thermodynamics is the study of the microscopic behaviors of thermodynamic systems using probability theory. Statistical thermodynamics, generally, provides a molecular level interpretation of thermodynamic quantities such as work, heat, free energy, and entropy. Statistical thermodynamics was born in 1870 with the work of Austrian physicist Ludwig Boltzmann, much of which was collectively published in Boltzmann's 1896 Lectures on Gas Theory.[1] Boltzmann's original papers on the statistical interpretation of thermodynamics, the H-theorem, transport theory, thermal equilibrium, the equation of state of gases, and similar subjects, occupy about 2,000 pages in the proceedings of the Vienna Academy and other societies. The term "statistical thermodynamics" was proposed for use by the American thermodynamicist Willard Gibbs in 1902. According to Gibbs, the term "statistical", in the context of mechanics, i.e. statistical mechanics, was first used by the Scottish physicist James Clerk Maxwell in 1871. Product highlight
OverviewThe essential problem in statistical thermodynamics is to determine the distribution of a given amount of energy E over N identical systems.[2] The goal of statistical thermodynamics is to understand and to interpret the measurable macroscopic properties of materials in terms of the properties of their constituent particles and the interactions between them. This is done by connecting thermodynamic functions to quantum-mechanic equations. Two central quantities in statistical thermodynamics are the Boltzmann factor and the partition function. HistoryIn 1738, Swiss physicist and mathematician Daniel Bernoulli published Hydrodynamica which laid the basis for the kinetic theory of gases. In this work, Bernoulli positioned the argument, still used to this day, that gases consist of great numbers of molecules moving in all directions, that their impact on a surface causes the gas pressure that we feel, and that what we experience as heat is simply the kinetic energy of their motion. In 1859, after reading a paper on the diffusion of molecules by Rudolf Clausius, Scottish physicist James Clerk Maxwell formulated the Maxwell distribution of molecular velocities, which gave the proportion of molecules having a certain velocity in a specific range. This was the first-ever statistical law in physics.[3] Five years later, in 1864, Ludwig Boltzmann, a young student in Vienna, came across Maxwell’s paper and was so inspired by it that he spent much of his long and distinguished life developing the subject further. Hence, the foundations of statistical thermodynamics were laid down in the late 1800s by those such as James Maxwell, Ludwig Boltzmann, Max Planck, Rudolf Clausius, and Willard Gibbs who began to apply statistical and quantum atomic theory to ideal gas bodies. Predominantly, however, it was Maxwell and Boltzmann, working independently, who reached similar conclusions as to the statistical nature of gaseous bodies. Yet, one most consider Boltzmann to be the "father" of statistical thermodynamics with his 1875 derivation of the relationship between entropy S and multiplicity Ω, the number of microscopic arrangements (microstates) producing the same macroscopic state (macrostate) for a particular system.[4] Classical thermodynamics vs. statistical thermodynamicsAs an example, from a classical thermodynamics point of view we might ask what is it about a thermodynamic system of gas molecules, such as ammonia NH3, that determines the free energy characteristic of that compound? Classical thermodynamics does not provide the answer. If, for example, we were given spectroscopic data, of this body of gas molecules, such as bond length, bond angle, bond rotation, and flexibility of the bonds in NH3 we should see that the free energy could not be other than it is. To prove this true, we need to bridge the gap between the microscopic realm of atoms and molecules and the macroscopic realm of classical thermodynamics. From physics, statistical mechanics provides such a bridge by teaching us how to conceive of a thermodynamic system as an assembly of units. More specifically, it demonstrates how the thermodynamic parameters of a system, such as temperature and pressure, are interpretable in terms of the parameters descriptive of such constituent atoms and molecules.[5] In a bounded system, the crucial characteristic of these microscopic units is that their energies are quantized. That is, where the energies accessible to a macroscopic system form a virtual continuum of possibilities, the energies open to any of its submicroscopic components are limited to a discontinuous set of alternatives associated with integral values of some quantum number. FundamentalsCentral topics covered in statistical thermodynamics include:
Lastly, and most importantly, the formal definition of entropy of a thermodynamic system from a statistical perspective is called statistical entropy is defined as: where
A common mistake is taking this formula as a hard general definition of entropy. This equation is valid only if each microstate is equally accessible (each microstate has an equal probability of occurring). RelatedIn the late 19th century, Ladislaus von Bortkiewicz, a Russian-born statistician, intrigued by the heating of cannons as they were fired attempted to statistically predict the overheating of an artillery battalion. His few trials showed that the metallic composition of cannon barrels in Poland varied too greatly at the time to effectively predict the outcomes of an entire battalion. See alsoReferences
Further reading
Categories: Thermodynamics | Statistical mechanics |
|||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Statistical_thermodynamics". A list of authors is available in Wikipedia. |





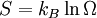
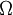 is the number of
is the number of 

