To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Lenoir cycleThe Lenoir cycle is an idealised thermodynamic cycle often utilized to model a pulse jet engine. It is based on the operation of an engine patented by Jean Joseph Etienne Lenoir in 1860. This engine is often thought of as the first commercially produced internal combustion engine. The absence of any compression process in the design leads to lower thermal efficiencies than the more well known Otto cycle and Diesel cycle. In the cycle, an ideal gas undergoes 1-2: Constant volume (isochoric) heat addition 2-3: Isentropic expansion. 3-1: Constant pressure (isobaric) heat rejection - compression to the volume at the start of the cycle. The expansion process is isentropic and hence involves no heat interaction. Energy is absorbed as heat during the constant volume process and rejected as heat during the constant pressure process. Product highlight
Constant volume heat addition (1-2)In the ideal gas version of the traditional Lenoir cycle, the first stage (1-2) involves the addition of heat in a constant volume manner. This results in the following for the first law of thermodynamics:
There is no work during the process because the volume is held constant:
and from the definition of constant volume specific heats for an ideal gas:
Where R is the ideal gas constant and γ is the ratio of specific heats (approximately 287 J/(kg·K) and 1.4 for air respectively). The pressure after the heat addition can be calculated from the ideal gas law: p2V2 = RT2 Isentropic expansion (2-3)The second stage (2-3) involves a reversible adiabatic expansion of the fluid back to its original pressure. It can be determined for an isentropic process that the second law of thermodynamics results in the following:
Where p3 = p1 for this specific cycle. The first law of thermodynamics results in the following for this expansion process: Constant pressure heat rejection (3-1)The final stage (3-1) involves a constant pressure heat rejection back to the original state. From the first law of thermodynamics we find: 3Q1 − 3W1 = U1 − U3. From the definition of work: As a result, we can determine the heat rejected as follows: The overall efficiency of the cycle is determined by the total work over the heat input, which for a Lenoir cycle equals: Cycle diagrams
|
|||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Lenoir_cycle". A list of authors is available in Wikipedia. | |||||||||||||||





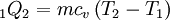
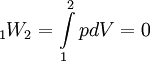
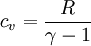
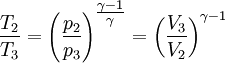
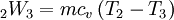 because for an adiabatic process:
because for an adiabatic process:
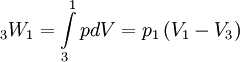 , we recover the following for the heat rejected during this process:
, we recover the following for the heat rejected during this process: 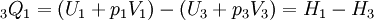 .
.
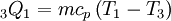 from the definition of constant pressure specific heats for an ideal gas:
from the definition of constant pressure specific heats for an ideal gas: 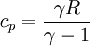 .
.
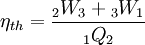 . Note that we gain work during the expansion process but lose some during the heat rejection process.
. Note that we gain work during the expansion process but lose some during the heat rejection process.


