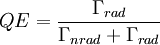To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Spontaneous emissionSpontaneous emission is the process by which a light source such as an atom, molecule, nanocrystal or nucleus in an excited state undergoes a transition to the ground state and emits a photon. Spontaneous emission of light or luminescence is a fundamental process that plays an essential role in many phenomena in nature and forms the basis of many applications, such as fluorescent tubes, television screens, plasma display panels, lasers and light emitting diodes. Product highlight
IntroductionIf a light source ('the atom') is in the excited state with energy E2, it may spontaneously decay to the ground state, with energy E1, releasing the difference in energy between the two states as a photon. The photon will have frequency ω and energy
where
If the number of light sources in the excited state is given by N, the rate at which N decays is:
where A21 is the rate of spontaneous emission. In the rate-equation A21 is a proportionality constant for this particular transition in this particular light source. The constant is referred to as the Einstein A coefficient, and has units s − 1[1]. The above equation can be solved to give:
where N(0) is the initial number of light sources in the excited state, t is the time and Γrad is the radiative decay rate of the transition. The number of excited states N thus decays exponentially with time, similar to radioactive decay. After one lifetime, the number of excited states decays to 36,8% of its original value ( TheoryQuantum mechanics explicitly prohibits spontaneous transitions. That is, using the machinery of ordinary first-quantized quantum mechanics and one computes the probability of spontaneous transitions from one stationary state to another, one finds that it is zero. In order to explain spontaneous transitions, quantum mechanics must be extended to a second-quantized theory, wherein the electromagnetic field is quantized at every point in space. Such a theory is known as a quantum field theory; the quantum field theory of electrons and electromagnetic fields is known as quantum electrodynamics. In quantum electrodynamics (or QED), the electromagnetic field has a ground state, the vacuum state, which can mix with the excited stationary states of the atom (for more information, see Ref. [2]). As a result of this interaction, the "stationary state" of the atom is no longer a true eigenstate of the combined system of the atom plus electromagnetic field. In particular, the electron transition from the excited state to the electronic ground state mixes with the transition of the electromagnetic field from the ground state to an excited state, a field state with one photon in it. Although there is only one electronic transition from the excited state to ground state, there are many ways in which the electromagnetic field may go from the ground state to a one-photon state. That is, the electromagnetic field has infinitely more degrees of freedom, corresponding to the directions in which the photon can be emitted. Equivalently, one might say that the phase space offered by the electromagnetic field is infinitely larger than that offered by the atom. Since one must consider probabilities that occupy all of phase space equally, the combined system of atom plus electromagnetic field must undergo a transition from electronic excitation to a photonic excitation; the atom must decay by spontaneous emission. The time the light source remains in the excited state thus depends on the light source itself as well as its environment. Rate of spontaneous emissionThe rate of spontaneous emission (i.e., the radiative rate) can be described by Fermi's golden rule.[2] The rate of emission depends on two factors: an 'atomic part', which describes the internal structure of the light source and a 'field part', which describes the density of electromagnetic modes of the environment. The atomic part describes the strength of a transition between two states in terms of transition moments. In a homogenous medium, such as free space, the rate of spontaneous emission is given by: where ω is the emission frequency, n is the index of refraction, μ12 is the transition dipole moment, Radiative and nonradiative decay: the quantum efficiencyIn the rate-equation above, it is assumed that decay of the number of excited states N only occurs under emission of light. In this case one speaks of full radiative decay and this means that the quantum efficiency is 100%. Besides radiative decay, which occurs under the emission of light, there is a second decay mechanism; nonradiative decay. To determine the total decay rate Γtot, radiative and nonradiative rates should be summed:
where Γtot is the total decay rate, Γrad is the radiative decay rate and Γnrad the nonradiative decay rate. The quantum efficiency (QE) is defined as the fraction of emission processes in which emission of light is involved: In nonradiative relaxation, the energy is released as phonons, more commonly known as heat. Nonradiative relaxation occurs when the energy difference between the levels is very small, and these typically occur on a much faster time scale than radiative transitions. For many materials (for instance, semiconductors), electrons move quickly from a high energy level to a meta-stable level via small nonradiative transitions and then make the final move down to the bottom level via an optical or radiative transition. This final transition is the transition over the bandgap in semiconductors. Large nonradiative transitions do not occur frequently because the crystal structure generally can not support large vibrations without destroying bonds (which generally doesn't happen for relaxation). Meta-stable states form a very important feature that is exploited in the construction of lasers. Specifically, since electrons decay slowly from them, they can be piled up in this state without too much loss and then stimulated emission can be used to boost an optical signal. Lifetime measurementsThe rate of emission can be measured with a photoluminescence lifetime measurement.[4] In lifetime measurements the decay of the number of light sources is probed by recording a photoluminescence decay curve. Time-correlated–single-photon counting is generally used to obtain decay curves. The decay curve is built from a histogram which shows the distribution of arrival times of single photons after many excitation-detection cycles. The histogram is modelled with a decay function from which the decay time of the process is deduced. In the simplest case the decay curve can be described by a single-exponential function. In a semi-logarithmic plot a single-exponential decay function results in a straight line. The slope of the straight line equals the total decay rate of the process. In many cases the decay curve is more complex than single-exponential.[5] In case of multi-exponential decay the process is not characterized by a single rate, but by a sum or a distribution of rates. It is a general problem to model these complex multi-exponential decay processes. Double and triple-exponential functions or functions with a particular distribution of rates are used. Controlling spontaneous emission: Purcell EffectThe rate of spontaneous emission depends partly on the environment of a light source. This means that by placing the light source in a special environment, the rate of spontaneous emission can be modified. In the 1950s E. Purcell discovered the enhancement of spontaneous emission rates of atoms when they are matched in a resonant cavity (the Purcell Effect)[6]. It has been predicted theoretically[7][8] that a 'photonic' material environment can control the rate of radiative recombination of an embedded light source. A main research goal is the achievement of a material with a complete photonic bandgap: a range of frequencies in which no electromagnetic modes exist and all propagation directions are forbidden. At the frequencies of the photonic bandgap, spontaneous emission of light is completely inhibited. Fabrication of a material with a complete photonic bandgap is a huge scientific challenge. For this reason photonic materials are being extensively studied. Many different kinds of systems in which the rate of spontaneous emission is modified by the environment are reported, including cavities, two,[9] and three-dimensional[10] photonic bandgap materials. See also
References
Categories: Laser science | Electromagnetic radiation |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Spontaneous_emission". A list of authors is available in Wikipedia. |





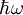 :
:
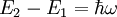 ,
,
 is Dirac's constant. The phase of the photon in spontaneous emission is random as is the direction the photon propagates in. This is not true for
is Dirac's constant. The phase of the photon in spontaneous emission is random as is the direction the photon propagates in. This is not true for 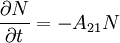 ,
,
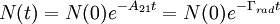 ,
,
 -time). The radiative decay rate
-time). The radiative decay rate 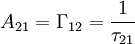 .
.
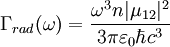
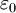 is the vacuum permitivity,
is the vacuum permitivity,