UCR scientists manipulate ripples in graphene, enabling strain-based graphene electronics
Study is first to experimentally quantify thermal contraction of graphene
Advertisement
graphene is nature's thinnest elastic material and displays exceptional mechanical and electronic properties. Its one-atom thickness, planar geometry, high current-carrying capacity and thermal conductivity make it ideally suited for further miniaturizing electronics through ultra-small devices and components for semiconductor circuits and computers.
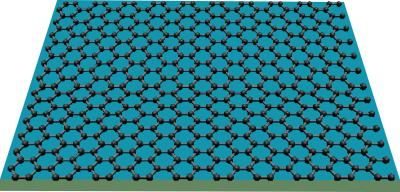
Graphene consists of carbon atoms only one atomic layer thick, with the unique characteristic that its electrons behave as if they have zero mass.
Lau lab, UC Riverside
But one of graphene's intrinsic features is ripples, similar to those seen on plastic wrap tightly pulled over a clamped edge. Induced by pre-existing strains in graphene, these ripples can strongly affect graphene's electronic properties, and not always favorably.
If the ripples can be controlled, however, they can be used to advantage in nanoscale devices and electronics, opening up a new arena in graphene engineering: strain-based devices.
UC Riverside's Chun Ning (Jeanie) Lau and colleagues now report the first direct observation and controlled creation of one- and two-dimensional ripples in graphene sheets. Using simple thermal manipulation, the researchers produced the ripples, and controlled their orientation, wavelength and amplitude.
"When the graphene sheets are stretched across a pair of parallel trenches, they spontaneously form nearly periodic ripples," Lau explained. "When these sheets are heated up, they actually contract, so the ripples disappear. When they are cooled down to room temperature, the ripples re-appear, with ridges at right angle to the edges of the trenches. This phenomenon is similar to what happens to a piece of thin plastic wrap that covers a container and heated in a microwave oven."
The unusual thermal contraction of graphene had been predicted theoretically, but Lau's lab is the first to demonstrate and quantify the phenomenon experimentally.
Because graphene is both an excellent conductor and the thinnest elastic membrane, the ripples could have profound implications for graphene-based electronics.
"This is because graphene's ability to conduct electricity is expected to vary with the local shape of the membrane," Lau said. "For instance, the ripples may produce effective magnetic fields that can be used to steer and manipulate electrons in a nanoscale device without an external magnet."
Lau, an associate professor of physics and a member of UCR's Center for Nanoscale Science and Engineering, and her colleagues examined the ripples' morphology using a scanning electron microscope and an atomic force microscope. They found that almost all the graphene membranes underwent dramatic morphological changes when heated, displaying significant alterations in the ripple geometry, a buckling of the graphene membrane, or both.
Their experimental system, which involved a stage inside a scanning electron microscope (SEM) with a built-in heater, thermometer and several electrical feed-throughs, enabled them to image graphene while it was being heated and explore the interplay between graphene's mechanical, thermal and electrical properties.
"Our result has important implications for controlling thermally induced stress in graphene electronics," Lau said. "Our ability to control and manipulate the ripples in graphene sheets represents the first step towards strain-based graphene engineering. We show that suspended graphene is almost invariably rippled, and this may need to be considered in the interpretation of a broad array of existing and future research."
Proposed to supplement or replace silicon as the main electronic material, graphene is a single layer of graphite. Even though graphite has been studied for decades, the single sheet first was isolated experimentally only in 2004. Graphene's structure is a two-dimensional honeycomb lattice of carbon atoms. Structurally, it is related to carbon nanotubes (tiny hollow tubes formed by rolling up sheets of graphene) and buckyballs (hollow carbon molecules that form a closed cage).
Original publication: Nature Nanotechnology 2009