A smart safe rechargeable zinc ion battery based on sol-gel transition electrolytes
Advertisement
The thermal runaway issue has been a long-standing obstacle impeding the development of high energy density and high power delivery batteries. These batteries would generate a lot of heat in ultrafast charge/discharge process or hazardous conditions, such as overcharging and short-circuit. To dissipate the heat accumulated in the batteries, physical safety designs such as fused disconnect switches, extinguishing agents, and shutdown current collectors have been employed. However, these approaches only provide a one-time protection. There is no provision for these strategies to spontaneously restore the original working condition of batteries once the temperature is cooled down. Therefore, intelligent and active internal safety strategies need to be designed for fabricating smart batteries with dynamic electrochemical performance and self-adaptive response to temperature.
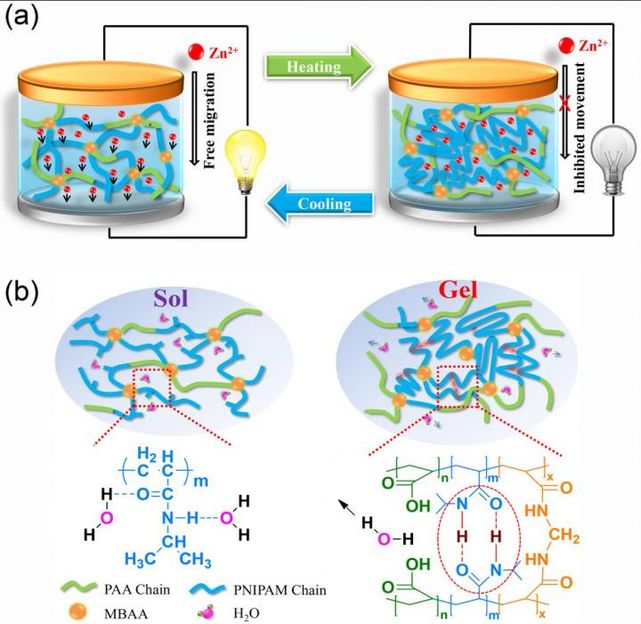
Schematic illustration of the thermoresponsive Zn/α-MnO2 batteries with reversible sol-gel transition electrolye.
Science China Press
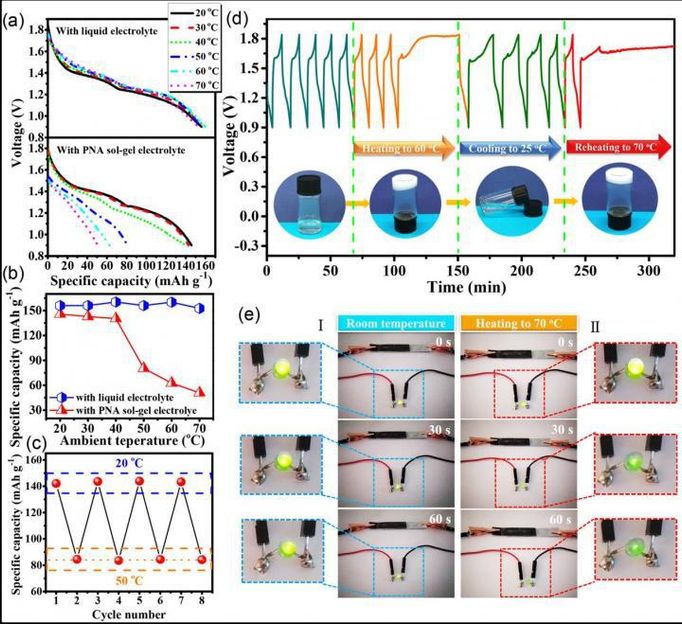
Dynamic electrochemical performance the thermoresponsive Zn/α-MnO2 batteries at different temperature.
Science China Press
Reversible sol-gel transition hydrogels have received abundant research interests owing to their smart responsibility to ambient temperature. They are normally in flowing liquid state at or below room temperature and can transform into stationary gels when heating above critical temperature. Moreover, this transition can be reversed after cooling down, displaying interesting temperature-dependent properties. Sol-gel transition polymer may potentially be good candidates for designing advanced batteries with intelligent thermal responsibility.
Recently, a research team led by Prof. Chunyi Zhi from City University of Hong Kong has successfully synthesized a temperature-sensitive sol-gel transition electrolyte comprising proton-incorporated poly(N-isopropylacrylamide-co-acrylic acid) (PNA) and incorporated it into a rechargeable Zn/α-MnO2 battery system. After heating above the low critical temperature, a gelation process occurs in the PNA sol-gel electrolyte and significantly inhibits the migration of zinc ions, leading to a decreased specific capacity and an increased internal resistance of the battery, thus shutting down the battery.
After cooling down, the transition is reversed to liquid state and an original electrochemical performance can be restored. More importantly, unlike traditional strategies, the sol-gel electrolyte endows the thermoresponsive battery with dynamic charge/discharge rate performance at different temperature, which enabled a "smart" thermal control for the battery. This work represents a feasible concept for self-protection batteries via reversible sol-gel transition.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.




























































