Electric Cloth: Flexible, wearable supercapacitors based on porous nanocarbon nanocomposites
Advertisement
Evening gowns with interwoven LEDs may look extravagant, but the light sources need a constant power supply from devices that are as well wearable, durable, and lightweight. Chinese scientists have manufactured fibrous electrodes for wearable devices that are flexible and excel by their high energy density. A microfluidic technology was key for the preparation of the electrode material was a microfluidic technology, as shown in the journal Angewandte Chemie.
© Wiley-VCH
Dresses sparkling light from hundreds of small LEDs may create eye-catching effects in ballrooms or on fashion shows. But wearable electronics can also mean sensors integrated in functional textiles to monitor, for example, water evaporation or temperature changes. Energy storage systems powering such wearable devices must combine deformability with high capacity and durability. However, deformable electrodes often fail in long-term operation, and their capacity lags behind that of other state-of-the-art energy storage devices.
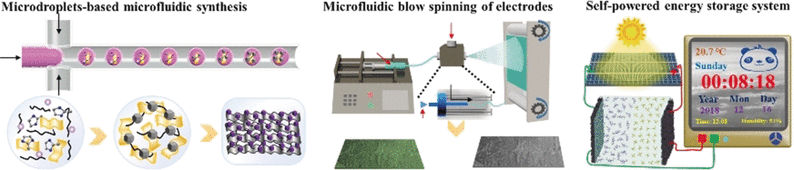
Electrode materials usually benefit from a fine balance of porosity, conductivity, and electrochemical activity. Material scientists Su Chen, Guan Wu, and their teams from the Nanjing Tech University, China, have looked deeper into the material demands for flexible electrodes and developed a porous hybrid material synthesized from two carbon nanomaterials and a metal–organic framework. The nanocarbons provided the large surface area and excellent electrical conductivity, and the metal–organic framework gave the porous structure and the electrochemical activity.
To make the electrode materials flexible for wearable applications, the micro-mesoporous carbon frameworks were spun into fibers with a thermoplastic resin by using an innovative blow-spinning machine. The resulting fibers were pressed into cloths and assembled into supercapacitors, although it turned out that another round of coating with the micro-mesoporous carbon frameworks further improved the electrode performances.
The supercapacitors made from these electrodes were not only deformable, but they could also harbor higher energy densities and larger specific capacitances than comparable devices. They were stable and endured more than 10,000 charge–discharge cycles. The scientists also tested them in practical applications such as smart color switching of LEDs in dresses and solar-cell-controlled powering of electronic devices integrated in functional clothing.
The authors pointed out that the microfluidic droplet-based synthesis was key to improving the performance of the electrode materials for wearable electronics. It was all about adjusting the perfect porous nanostructure, they argued.
Original publication
Other news from the department science
These products might interest you

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.
Topic world Sensor technology
Sensor technology has revolutionized the chemical industry by providing accurate, timely and reliable data across a wide range of processes. From monitoring critical parameters in production lines to early detection of potential malfunctions or hazards, sensors are the silent sentinels that ensure quality, efficiency and safety.