Army scientists look inside batteries with a molecular eye
Advertisement
Scientists are closer to understanding exactly what happens inside batteries that make them prone to fire, thanks to a molecular eye of sorts.
Scientists at the U.S. Army Combat Capabilities Development Command's Army Research Laboratory teamed with researchers from the U.S. Department of Energy's Pacific Northwest National Laboratory to study chemical reactions that occur when two key components of battery interface, forming a critical component in battery commonly known as the solid-electrolyte-interphase, or SEI.
Understanding the chemistry and formation mechanism of this SEI holds the key to unlocking future better batteries, reseachers said. This new research, enabled by a technique that serves as a molecular eye, presents a dynamic picture of the chemistry and structure of SEI.
These properties are known to influence the battery charge-discharge rate, especially at low temperature, safety and cycle life, according to Army scientist Dr. Oleg Borodin, a researcher with a team focusing on electrochemistry.
"SEIs are critical for battery properties but elusive to characterize," said Dr. Kang Xu, a principal investigator on this research project. "They dictate how fast a battery could be charged for Warfighters in order to improve operational capabilities as well as preventing slow and abrupt battery failure during mission. But like dark matters, everyone knows they exist but no one knows how they work."
Nearly four years ago, ARL scientists began collaborating with experts "who are not battery people," Xu said, but have unique expertise in advanced characterization techniques. He said Army researchers laid down the basic challenges faced in understanding SEIs, and asked for help. This collaboration resulted in a series of groundbreaking work in SEI, and some of the results have already been published in Nature Chemistry and Nature Nanotechnology in 2018 and 2019.
Their latest work is featured in the article, Real-time mass spectrometric characterization of the solid-electrolyte interphase of a lithium-ion battery, published in Nature Nanotechnology.
Scientists from the Environmental Molecular Sciences Laboratory and Pacific Northwest National Laboratory developed this technique, an in situ liquid secondary ion mass spectrometry and partnered with Army scientists to apply this technique on investigating the chemical workings at the electrode-electrolyte interface on a molecular level when the battery was charged in its first hour. They monitored the formation of the SEI and its chemistry variation. The approach allowed them to map the chemical reactions as they occurred. When combined with molecular dynamics simulations, their work revealed something that had only been speculated before.
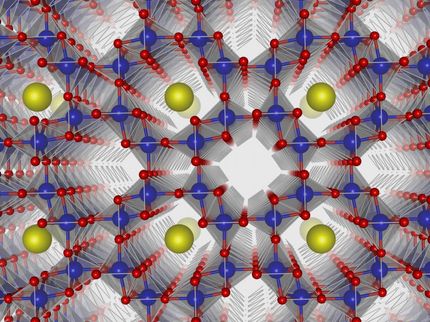
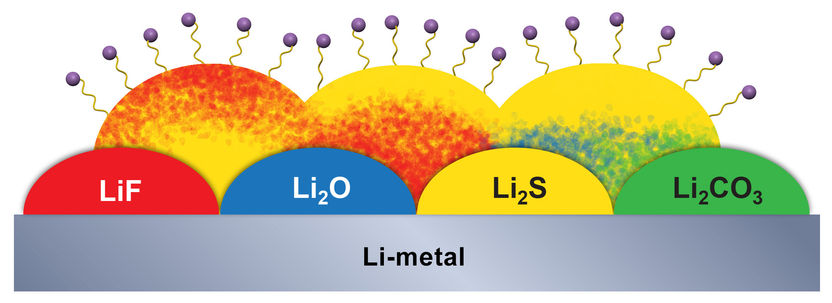
During initial battery charging, the battery forms an electric double layer at the electrode/electrolyte interface. The creation of this double layer leads to fine structural and chemical variations of SEI, which will eventually dictate the performance of battery itself. The molecular-level understanding of the interface could serve as insightful guide to Army scientists' efforts in designing better batteries.
These researchers find that before any interphasial chemistry occurs (during the initial charging), an electric double layer forms at the electrode/electrolyte interface due to the self-assembly of solvent molecules. The formation of the double layer is directed by Li+ and the electrode surface potential. The structure of this double layer predicts the eventual interphasial chemistry; in particular, the negatively charged electrode surface repels salt anions from the inner layer and results in an inner SEI that is thin, dense and inorganic in nature. It is this dense layer that is responsible for conducting Li+ and insulating electrons, the main functions of the SEI. An electrolyte-permeable and organic-rich outer layer appears after the formation of the inner layer. In the presence of a highly concentrated, fluoride-rich electrolyte, the inner SEI layer has an elevated concentration of LiF due to the presence of anions in the double layer. These real-time nanoscale observations will be helpful in engineering better interphases for future batteries.
For decades, scientists tried to study the SEIs in lithium-ion batteries, with limited success due to the absence of a technique that would allow them to see battery operations at a tiny scale. Such nanoscale observations on a molecular level are needed to understand the chemistry at the interface.
In 2017, Army researchers partnerd with the University of Maryland developed for the first time a lithium-ion battery that uses a water-salt solution as its electrolyte and reaches the 4.0 volt mark desired for household electronics, such as laptop computers, without the fire and explosive risks associated with some commercially available non-aqueous lithium-ion batteries. The majority of commerically-available batteries are different from the aqueous batteries invented by this team. Understanding the SEI could lead to incrementally improving current technology as an immediate solution for many Army applications.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.