Discovery of New Solid Catalysts for Water Electrolysis
Advertisement
green hydrogen - produced from water electrolysis by using sustainable electricity - is getting more attention due to its potential to be used as energy carrier as well as building block for various industrial processes. Among both half-reactions of water electrolysis, Oxygen Evolution Reaction (OER) is kinetically more challenging and it requires advances in the development of innovative electrocatalysts.
Harun Tüysüz, MPI für Kohlenforschung
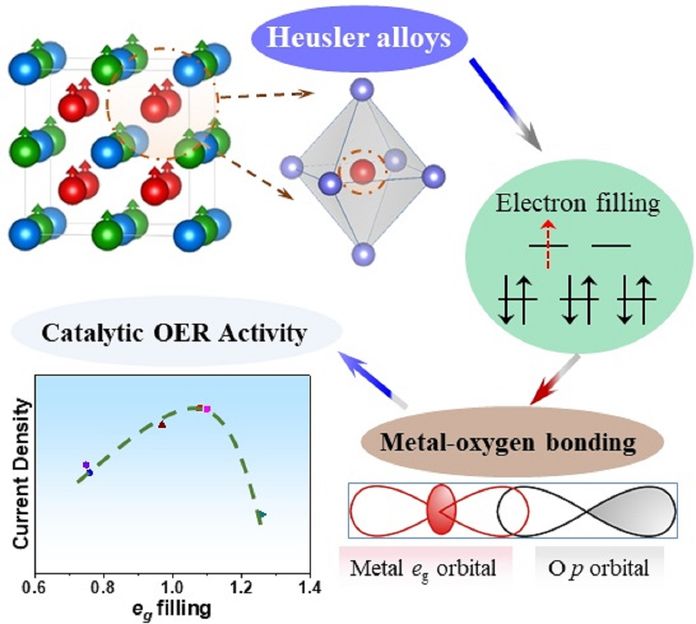
PD Dr. Harun Tüysüz (Max-Planck-Institut für Kohlenforschung), Prof. Dr. Claudia Felser (Max-Planck-Institut für Chemische Physik fester Stoffe) and co-workers have discovered a new type of OER electrocatalyst. A variety of Co2YZ type Heusler compounds with tunable physicochemical properties and well-defined topological surfaces were designed and demonstrated to effectively split water into oxygen and hydrogen. The systematic electrocatalytic investigation of the KOFO team proved a solid correlation between electron filling of d-orbitals of cobalt centers and their OER activities. The materials showed a volcano-shaped activity curve where the higher catalytic current was obtained for eg orbital filling approaching unity.
This work demonstrates proof of concept implementation of Heusler compounds as a new class of OER electrocatalysts, and the effect of orbital occupation on their catalytic performances.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
































































