New ammonia production method could open doors for alternative fuel, energy storage
A solution to climate change could be sitting under your bathroom sink
Advertisement
ammonia, a compound of nitrogen and hydrogen (NH3) that is commonly known for its use as a household cleaner, could be used as an alternative fuel for vehicles or as a potential energy storage medium. However, traditional methods of producing ammonia from nitrogen oxide (NO) on a large scale are not energy efficient, and some of the more recent efforts to produce ammonia from an NO reduction reaction have low ammonia yields. Now, researchers from Tsinghua University Press in China have developed an eco-friendly, energy-efficient method for producing ammonia through an electrocatalytic NO reduction reaction.
The assembled Zn–NO battery shows superior performance for ammonia production.
Nano Research Energy, Tsinghua University Press
The work was published in the journal Nano Research Energy on July 07.
“The industrial-level NH3 production is still heavily relying on the Haber–Bosch process, requiring drastic reaction conditions due to the sluggish kinetics, and the energy input aspect of the process inevitably results in a large amount of greenhouse gas emissions,” said corresponding author Xijun Liu of the School of Resource, Environments and Materials at Guangxi University in Nanning, China. His reference to the Haber-Bosch process alludes to one of the first — and, since its development in the early 1900s, one of the most common — NH3 production processes.
Because of these drawbacks, previous research has explored electrocatalytic — simply, a catalyst in an electromagnetic reaction — ammonia production in water because of its zero-carbon output using noble metal-based materials, carbon-based materials and single-atom catalysts. However, the yield rate was far lower than the U.S. Department of Energy target, according to the researchers.
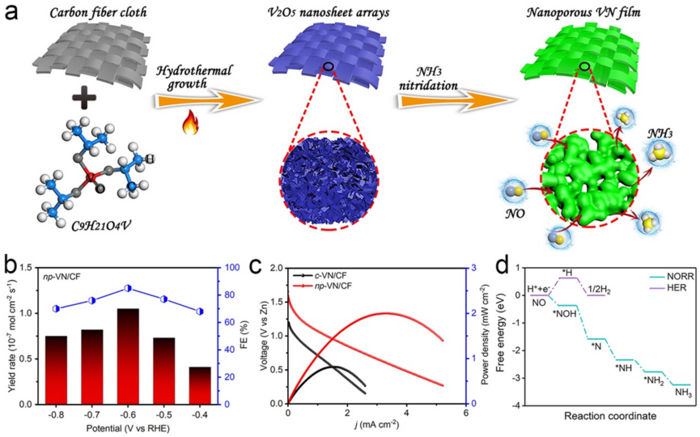
The researchers explored NO reaction reduction (NORR) to ammonia using the synthesis of nanoporous vanadium nitride (VN) film supported on carbon fiber cloth (written as np-VN/CF). They tested the new method, the novelty of which lies with using existing materials of VN film in a new way and for a new purpose, with a zinc-nitrogen oxide battery.
“This designed catalyst shows a high power density and a high corresponding ammonia yield rate when used as the cathode in a home-assembled Zn–NO battery,” Liu said. “We also found that the faraday efficiency ¾ or how well the electrons, or charge, are transferred in an electrochemical transformation ¾ was improved. The achieved NORR performance metrics are comparable to the recently reported best results.”
The high faraday efficiency can be attributed in part to the fact that part of the nitrogen was “doped” into carbon fiber cloth, making it more likely to be highly conductive and therefore favor the charge transfer.
To test the method with the battery, the researchers bubbled NO feeding gas into the cathode — or negatively charged portion of a battery — chamber of the battery, which was separated from the positive charge by a bipolar membrane. The obtained Zn-NO battery performance outperformed previously reported results, according to the researchers. The measurements attained from the experiments were tested in a three-electrode system and compared with the NORR activity of a commercial VN powder—as opposed to film—cast onto a carbon fiber cloth. The VN film version outperformed the powder version, which had been used in previous research, in all of the measurements taken. The researchers also performed density functional theory computations to offer an in-depth insight into NORR.
“Our work shows the potential application of nanoporous materials for high-performance electrochemical NH3 production,” Liu said.
Next steps for the research would include efforts to scale the proof-of-concept demonstrated in this experiment.
“This work further confirms that the electroreduction of NO is a promising strategy for ambient ammonia synthesis that should be continuously developed,” Liu said.
































































