Recharging on stable, amorphous silicon
Advertisement
Next-generation anodes for lithium ion batteries will probably no longer be made of graphite. silicon, which is a related material, can provide a much higher capacity than graphite, but its crystallinity poses problems. Chinese scientists have introduced a porous silicon form that is amorphous, not crystalline, and has the potential to outstrip the other materials in rechargeable battery applications.
© Wiley-VCH
Although carbon in its graphite form is the most common anode material today in lithium ion batteries, its capacity is relatively low. Other long-standing issues of lithium ion batteries are poor cycle life, increasing internal resistance with cycling, ageing, and safety concerns. Silicon offers a theoretical capacity almost ten times higher than that of graphite. However, silicon does not like cycling: Its crystalline structure expands and shrinks with every charge-discharge cycle, which leads to pulverization and capacity loss. Jian Yang and his team at Shangdong University in China have now a prepared a porous amorphous silicon modification that compensates for the disadvantages.
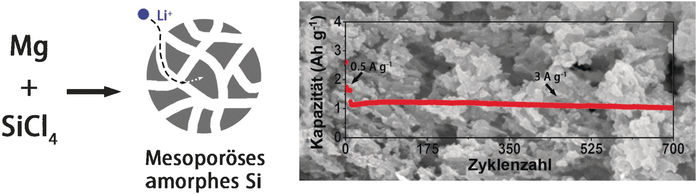
Yang said that investigation of the amorphous state was the logical consequence because silicon would loose crystallinity anyway. The authors wrote: "As silicon eventually becomes amorphous during electrochemical lithiation/delithiation, the attempt to use amorphous silicon ... from the beginning draws intense interest." On the other hand, amorphous silicon structures are rather difficult to prepare and the preparation conditions have to be carefully chosen. The scientists eventually came up with a relatively simple process, using safe substances as the starting materials, as they pointed out. For example, they used cheap and common glyme as the solvent, and liquid silicon tetrachoride as the silicon precursor, which would be easier to handle than other substances. All this makes their procedure "very attractive for the mass production," as they put it.
The resulting porous amorphous silicon material exhibited excellent electrochemical characteristics with a capacity three times better than graphite, and much longer cycling stability than crystalline silicon. Yang and his colleagues explained this stability by the presence of large, solvent-filled pores in the material and by the partial oxidation of the silicon surface in air. And there is more potential for the future. Yang proposes that a pinch of carbon in the structure would even further enhance its electrochemical performance.
Original publication
Jian Yang et al.; "Mesoporous Amorphous Silicon: A Simple Synthesis of a High-Rate and Long-Life Anode Material for Lithium-Ion Batteries"; Angewandte Chemie; 2016
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.