Nanodiamonds in Oil
Researchers isolate cyclohexamantane from petroleum
09-May-2003
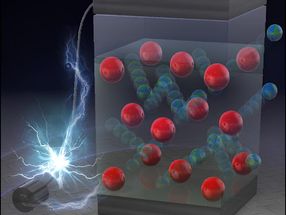
Even though their modest 10-20 carats will not impress your girlfriend, cyclohexamantane molecules are, despite their nanometer-scale dimensions, a part of the diamond family. They belong to the class of molecules known as diamondoids, unusual hydrocarbons whose cage-like carbon atom arrangements correspond to sections of the crystal structure of diamond. Researchers from oil company ChevronTexaco recently found these diamonds/order_t/'>nanodiamonds in crude oil.
The simplest diamondoid is called adamantane (Greek for diamond) and consists of ten carbon atoms, whose arrangement corresponds exactly to a single "cell" of the diamond structure. Jeremy E. P. Dahl and his co-workers have been able to identify over 20 different higher diamondoids, which consist of up to eleven adamantane units. Dahl and researchers from ChevronTexaco, several American and European Universities, Pfizer and BP, have now characterized and verified the structure of a special representative of the diamondoids, cyclohexamantane. The cyclohexamantane framework consists of 26 carbon atoms, as though six adamantane units were fused into a disk-shaped molecule. A total of 30 hydrogen atoms occupy the corners of the carbon cage.
Jewelers may not be able to muster up much enthusiasm for diamondoids, but scientists are fascinated by nanodiamonds, which not only share a common structure with their macroscopic cousins, but also possess their extraordinary strength and stability. At the same time, the different structures of the molecules provide an enormous structural diversity. In principle, the corner atoms could also be equipped with very different functional groups, which would result in great chemical versatility. Diamondoids are thus ideal building blocks for nanotechnology, and pharmacological applications are also possible.
"It has thus far not been possible to produce cyclohexamantane by synthetic routes," reports Dahl. "Petroleum is the only known source." "How cyclohexamantane is formed remains unclear, however, the reaction pathway which leads to their Formation, could also have led to larger diamondoids, even to microcrystalline diamonds. We are currently testing this hypothesis."
Most read news
Topics
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.