UCR chemists prepare molecules that accelerate chemical reactions for manufacturing drugs
New molecules help make stable catalysts that work at room temperature
Advertisement
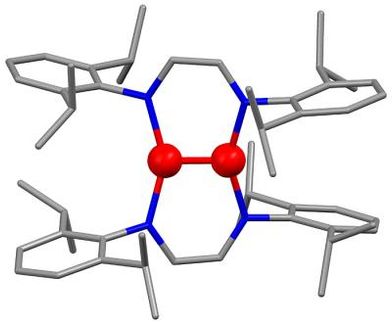
Chemists at the University of California, Riverside have synthesized a new class of carbenes that is expected to have wide applications in the pharmaceutical industry, ultimately resulting in a reduction in the price of drugs.
Called cyclic alkyl amino carbenes or CAACs, the molecules attach themselves to metals, such as palladium, to form highly efficient catalysts that allow chemical transformations otherwise considered impossible. The carbenes modulate the properties of the metals to which they are bound and can facilitate and speed up reactions involving their use. Study results appear in the Angewandte Chemie International Edition, and were published online Aug. 1.
In their paper, the UCR chemists discuss a set of chemical reactions involving the use of catalysts other than those that are CAAC-based. The authors note that these catalysts need strong heating to be effective. They add that the CAAC-based catalysts, on the other hand, can be used not only at room temperature but also in smaller amounts than is necessary for the other catalysts.
Because nitrogen atoms stabilize a carbene when they are adjacent to it, chemists believed until now that two nitrogen atoms were necessary in a carbene to make efficient catalysts. But having two nitrogen atoms also imposes structural limitations at the center of the carbene. The carbenes synthesized by the UCR chemists has only one nitrogen atom, which lends the molecule a far more flexible structure. In effect, the carbenes are bigger at the metallic center of the catalyst, a feature that improves the efficiency of the catalyst.
Most read news
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.