Surface modification of gold nanorods inside polymer beads
'Gold nanorods can be made stable and soluble in both polar and non-polar solvents', say US scientists
Advertisement
A team led by Qun Huo at the University of Central Florida, Orlando, have developed a method to replace the ionic surfactant layer on a gold nanorod surface with a more stable thiol ligand. This is carried out by a place exchange reaction conducted inside an ionic exchange polymer bead.
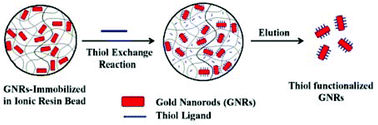
The gold nanorods were first loaded into porous ionic exchange polymer beads. The trapped gold nanorods were then exchanged for a bifunctional thiol ligand inside the polymer beads leading to successful surface modification of the gold nanorods with a thiol ligand protecting layer. This process is called a place exchange reaction.
Huo explains that place exchange reactions in solution are often problematic when applied to gold nanorods. This is because such direct modification in solution usually leads to an irreversible agglomeration of the gold nanorods. Huo's method prevents this by controlling the thiol ligand place exchange reaction inside the pores of a polymer bead. 'The trapping of gold nanorods inside polymer beads temporarily allows sufficient time for a stable thiol monolayer to form on the gold nanorod surface,' adds Huo.
The resulting gold nanorods show good solubility and stability against aggregation in both polar and non-polar solvents. These types of gold nanorods are known to possess many interesting optical properties and have potential application in biological systems.
Original publication: Qiu Dai, Janelle Coutts, Jianhua Zou and Qun Huo; Chem. Commun., 2008.
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.