Oxygen going for gold
Advertisement
Buddie Mullins and co-workers from the University of Texas, in the US, have performed the oxidative dehydrogenation of amines using heterogeneous gold catalysts with chemisorbed atomic oxygen on the gold surface.
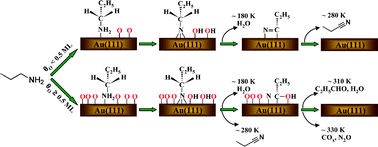
Mullins and his team show that gold covered with atomic oxygen, in the absence of any support, can facilitate the selective oxidation of propylamine to propionitrile and propionaldehyde at low temperatures. In addition the products are tuneable depending on the coverage of the oxygen.
Research into reactions catalysed by gold is a fast growing area, and there is great interest in understanding the effects of the support, the particle size, shape and the structural sensitivity of gold catalysts on reactivity and selectivity.
In the future, Mullins and his team hope to compare the catalytic ability of oxygen pre-covered gold single-crystals, size selected gold clusters on metal oxide wafers and gold single-crystals supported on metal oxide clusters, on a family of aliphatic or aromatic amines. This could help to elucidate mechanistic details and lead to more efficient gold-based catalysts for commercial applications.
Challenges facing research in oxidative catalysis by gold involve understanding the mechanism by which molecular oxygen is activated. At present this is difficult as reaction intermediates in oxygen activation are hard to isolate and study. However, ‘computational studies of this complex surface chemistry would be very useful,’ says Mullins.
Original article: C. Buddie Mullins et al., Chem. Commun., 2009.
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.