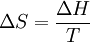To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Melting
Melting is a process that results in the phase change of a substance from a solid to a liquid. The internal energy of a solid substance is increased (typically by the application of heat) to a specific temperature (called the melting point) at which it changes to the liquid phase. An object that has melted completely is molten. The melting point of a substance is equal to freezing point. Additional recommended knowledge
Molecular vibrationsWhen the internal energy of a gas is increased by the application of an external energy source, the molecular vibrations of the substance increases. As these vibrations increase, the substance becomes more and more ordered. Constant temperatureSubstances melt at a constant temperature, the melting point. Further increases in temperature (even with continued application of energy) do not occur until the substance is molten. The thermodynamics of meltingFrom a thermodynamics point of view, at the melting point the change in Gibbs free energy (ΔG) of the Material is zero, because the enthalpy (H) and the entropy (S) of the material are increasing (ΔH,ΔS > 0). Melting phenomenon happens when the Gibbs free energy of the liquid becomes lower than the solid for that material. At various pressures this happens at a specific temperature. It can also be shown that:
The "T","ΔS", and "ΔH" in the above are respectively the temperature at the melting point, change of entropy of melting, and the change of enthalpy of melting. Books
Other meaningsLook up melting in Wiktionary, the free dictionary.
In genetics, melting DNA means to separate the double-stranded DNA into two single strands by heating or the use of chemicals.
See also
Categories: Metallurgy | Phase changes |
||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Melting". A list of authors is available in Wikipedia. | ||||||||||||||||||||||||||||||||||||||||||||