Nanotubes can solder themselves
Advertisement
University of Illinois researchers have developed a way to heal gaps in wires too small for even the world’s tiniest soldering iron.
Led by electrical and computer engineering professor Joseph Lyding and graduate student Jae Won Do, the Illinois team published its results in the journal Nano Letters.
Carbon nanotubes are like tiny hollow wires of carbon just 1 atom thick – similar to graphene but cylindrical. Researchers have been exploring using them as transistors instead of traditional silicon, because carbon nanotubes are easier to transport onto alternate substrates, such as thin sheets of plastic, for low-cost flexible electronics or flat-panel displays.
Carbon nanotubes themselves are high-quality conductors, but creating single tubes suitable to serve as transistors is very difficult. Arrays of nanotubes are much easier to make, but the current has to hop through junctions from one nanotube to the next, slowing it down. In standard electrical wires, such junctions would be soldered, but how could the gaps be bridged on such a small scale?
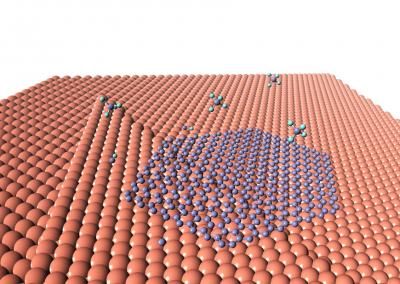
“It occurred to me that these nanotube junctions will get hot when you pass current through them,” said Lyding, “kind of like faulty wiring in a home can create hot spots. In our case, we use these hot spots to trigger a local chemical reaction that deposits metal that nano-solders the junctions.”
Lyding’s group teamed with Eric Pop, an adjunct professor of electrical and computer engineering, and John Rogers, Swanlund professor in materials science and engineering, experts on carbon nanotube synthesis and transfer, as well as chemistry professor Greg Girolami. Girolami is an expert in a process that uses gases to deposit metals on a surface, called chemical vapor deposition (CVD).
The nano-soldering process is simple and self-regulating. A carbon nanotube array is placed in a chamber pumped full of the metal-containing gas molecules. When a current passes through the transistor, the junctions heat because of resistance as electrons flow from one nanotube to the next. The molecules react to the heat, depositing the metal at the hot spots and effectively “soldering” the junctions. Then the resistance drops, as well as the temperature, so the reaction stops. (See video for demonstration of the process.)
The nano-soldering takes only seconds and improves the device performance by an order of magnitude – almost to the level of devices made from single nanotubes, but much easier to manufacture on a large scale.
“It would be easy to insert the CVD process in existing process flows,” Lyding said. “CVD technology is commercially available off-the-shelf. People can fabricate these transistors with the ability to turn them on so that this process can be done. Then when it’s finished they can finish the wiring and connect them into the circuits. Ultimately it would be a low-cost procedure.”
Now, the group is working to refine the process.
“We think we can make it even better,” Lyding said. “This is the prelude, we hope, but it’s actually quite significant.”