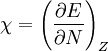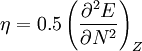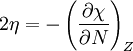To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
HSAB theoryThe HSAB concept is an acronym for 'hard and soft acids and bases'. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical species. 'Hard' applies to species which are small, have high charge states (the charge criterion applies mainly to acids, to a lesser extent to bases), and are weakly polarizable. 'Soft' applies to species which are big, have low charge states and are strongly polarizable.[1] The theory is used in contexts where a qualitative, rather than quantitative description would help in understanding the predominant factors which drive chemical properties and reactions. This is especially so in transition metal chemistry, where numerous experiments have been done to determine the relative ordering of ligands and transition metal ions in terms of their hardness and softness. HSAB theory is also useful in predicting the products of metathesis reactions. Quite recently it has been shown that even the sensitivity and performance of explosive materials can be explained on basis of HSAB theory [2] Ralph Pearson introduced the HSAB principle in the early 1960s[3][4] as an attempt to unify inorganic and organic reaction chemistry.[5]. Product highlight
TheoryThe gist of this theory is that soft acids react faster and form stronger bonds with soft bases, whereas hard acids react faster and form stronger bonds with hard bases, all other factors being equal.[6] The classification in the original work was mostly based on equilibrium constants for reaction of two Lewis bases competing for a Lewis acid. Hard acids and hard bases tend to have:
Examples of hard acids are: H+, alkali ions, Ti4+, Cr3+, Cr6+, BF3. Examples of hard bases are: OH–, F–, Cl–, NH3, CH3COO–, CO32–. The affinity of hard acids and hard bases for each other is mainly ionic in nature. Soft acids and soft bases tend to have:
Examples of soft acids are: CH3Hg+, Pt4+, Pd2+, Ag+, Au+, Hg2+, Hg22+, Cd2+, BH3. Examples of soft bases are: H–, R3P, SCN–, I–. The affinity of soft acids and bases for each other is mainly covalent in nature.
Borderline cases are also identified: borderline acids are trimethylborane, sulfur dioxide and ferrous Fe2+, cobalt Co2+ and lead Pb2+ cations. Borderline bases are: aniline, pyridine, nitrogen N2 and the azide, bromine, nitrate and sulphate anions. Generally speaking, acids and bases interact and the most stable interactions are hard-hard (ionogenic character) and soft-soft (covalent character). An attempt to quantify the 'softness' of a base consists in determining the equilibrium constant for the following equilibrium:
Where CH3Hg+ (methylmercury ion) is a very soft acid and H+ (proton) is a hard acid, which compete for B (the base to be classified). Some examples illustrating the effectiveness of the theory:
Chemical hardness
In 1983 Pearson together with Robert Parr extended the qualitative HSAB theory with quantitative chemical hardness (η) defined as [7]: with When the electronegativity (χ) as the Mulliken scale: is the first derivative in a plot of energy then the chemical hardness is simply the second derivative: Hardness and electronegativity are related as: and in this sense hardness is a measure for resistance to deformation or change. Likewise a value of zero denotes maximum softness. In a compilation of hardness values only that of the hydride anion deviates. Another discrepancy noted in the original 1983 article are the apparent higher hardness of Tl3+ compared to Tl+. Kornblum's ruleAn application of HSAB theory is the so-called Kornblum's rule which states that in reactions with ambident nucleophiles, the more electronegative atom reacts when the reaction mechanism is SN1 and the less electronegative one in a SN2 reaction. This rule (established in 1954) [8] actually predates HSAB theory but in HSAB terms its explanation is that in a SN1 reaction the carbocation (a hard acid) reacts with a hard base (high electronegativity) and that in a SN2 reaction tetravalent carbon (a soft acid) reacts with ditto soft bases. References
See alsoCategories: Acid-base chemistry | Inorganic chemistry |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "HSAB_theory". A list of authors is available in Wikipedia. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||





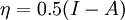
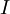 the
the 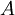 the
the 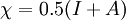
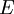 versus the amount of
versus the amount of 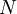 with fixed
with fixed 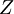 in an atom or molecule:
in an atom or molecule: