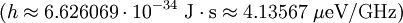To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Electromagnetic spectrum
The electromagnetic (EM) spectrum is the range of all possible electromagnetic radiation. The "electromagnetic spectrum" (usually just spectrum) of an object is the characteristic distribution of electromagnetic radiation from that object. The electromagnetic spectrum, extends from below the frequencies used for modern radio (at the long-wavelength end) through gamma radiation (at the short-wavelength end), covering wavelengths from thousands of kilometres down to a fraction the size of an atom. In our universe the short wavelength limit is likely to be in the vicinity of the Planck length, and the long wavelength limit is the size of the universe itself (see physical cosmology), though in principle the spectrum is infinite and continuous. Product highlight
Range of the spectrum
The spectrum covers EM energy having wavelengths from thousands of kilometers down to fractions of the size of an atom. Frequencies of 30 Hz and below can be important in certain stellar nebulas [1] and frequencies as high as 2.9 * 1027 Hz have been detected from astrophysical sources [2] Electromagnetic energy at a particular wavelength λ (in vacuum) has an associated frequency f and photon energy E. Thus, the electromagnetic spectrum may be expressed equally well in terms of any of these three quantities. They are related by the equations:
Where So, high-frequency electromagnetic waves have a short wavelength and high energy; low-frequency waves have a long wavelength and low energy. When light waves (and other electromagnetic waves) enter a medium, their wavelength is reduced. Wavelengths of electromagnetic radiation, no matter what medium they are travelling through, are usually quoted in terms of the vacuum wavelength, although this is not always explicitly stated. Generally, EM radiation is classified by wavelength into electrical energy, radio, microwave, infrared, the visible region we perceive as light, ultraviolet, X-rays and gamma rays. The behavior of EM radiation depends on its wavelength. Higher frequencies have shorter wavelengths, and lower frequencies have longer wavelengths. When EM radiation interacts with single atoms and molecules, its behavior depends on the amount of energy per quantum it carries. Electromagnetic radiation can be divided into octaves — as sound waves are.[4] Spectroscopy can detect a much wider region of the EM spectrum than the visible range of 400 nm to 700 nm. A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. It is widely used in astrophysics. For example, many hydrogen atoms emit radio waves which have a wavelength of 21.12 cm. Types of radiationWhile the classification scheme is generally accurate, in reality there is often some overlap between neighboring types of electromagnetic energy. For example, SLF radio waves at 60 Hz may be received and studied by astronomers, or may be ducted along wires as electric power. Also, some low-energy gamma rays actually have a longer wavelength than some high-energy X-rays. This is possible because "gamma ray" is the name given to the photons generated from nuclear decay or other nuclear and subnuclear processes, whereas X-rays on the other hand are generated by electronic transitions involving highly energetic inner electrons. Therefore the distinction between gamma ray and X-ray is related to the radiation source rather than the radiation wavelength. Generally, nuclear transitions are much more energetic than electronic transitions, so usually, gamma-rays are more energetic than X-rays. However, there are a few low-energy nuclear transitions (e.g. the 14.4 keV nuclear transition of Fe-57) that produce gamma rays that are less energetic than some of the higher energy X-rays. Radio frequencyRadio waves generally are utilized by antennas of appropriate size (according to the principle of resonance), with wavelengths ranging from hundreds of meters to about one millimeter. They are used for transmission of data, via modulation. Television, mobile phones, MRI, wireless networking and amateur radio all use radio waves. Radio waves can be made to carry information by varying a combination of the amplitude, frequency and phase of the wave within a frequency band and the use of the radio spectrum is regulated by many governments through frequency allocation. When EM radiation impinges upon a conductor, it couples to the conductor, travels along it, and induces an electric current on the surface of that conductor by exciting the electrons of the conducting material. This effect (the skin effect) is used in antennas. EM radiation may also cause certain molecules to absorb energy and thus to heat up; this is exploited in microwave ovens. MicrowavesThe super high frequency (SHF) and extremely high frequency (EHF) of Microwaves come next up the frequency scale. Microwaves are waves which are typically short enough to employ tubular metal waveguides of reasonable diameter. Microwave energy is produced with klystron and magnetron tubes, and with solid state diodes such as Gunn and IMPATT devices. Microwaves are absorbed by molecules that have a dipole moment in liquids. In a microwave oven, this effect is used to heat food. Low-intensity microwave radiation is used in Wi-Fi. The average microwave oven in active condition is, in close range, powerful enough to cause interference with poorly shielded electromagnetic fields such as those found in mobile medical devices and cheap consumer electronics. Terahertz radiationTerahertz radiation is a region of the spectrum between far infrared and microwaves. Until recently, the range was rarely studied and few sources existed for microwave energy at the high end of the band (sub-millimetre waves or so-called terahertz waves), but applications such as imaging and communications are now appearing. Scientists are also looking to apply Terahertz technology in the armed forces, where high frequency waves might be directed at enemy troops to incapacitate their electronic equipment. Infrared radiationThe infrared part of the electromagnetic spectrum covers the range from roughly 300 GHz (1 mm) to 400 THz (750 nm). It can be divided into three parts:
Visible radiation (light)Above infrared in frequency comes visible light. This is the range in which the sun and stars similar to it emit most of their radiation. It is probably not a coincidence that the human eye is sensitive to the wavelengths that the sun emits most strongly. Visible light (and near-infrared light) is typically absorbed and emitted by electrons in molecules and atoms that move from one energy level to another. The light we see with our eyes is really a very small portion of the electromagnetic spectrum. A rainbow shows the optical (visible) part of the electromagnetic spectrum; infrared (if you could see it) would be located just beyond the red side of the rainbow with ultraviolet appearing just beyond the violet end. EM radiation with a wavelength between approximately 400 nm and 700 nm is detected by the human eye and perceived as visible light. Other wavelengths, especially nearby infrared (longer than 700 nm) and ultraviolet (shorter than 400 nm) are also sometimes referred to as light, especially when the visibility to humans is not relevant. If radiation having a frequency in the visible region of the EM spectrum reflects off of an object, say, a bowl of fruit, and then strikes our eyes, this results in our visual perception of the scene. Our brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this not-entirely-understood psychophysical phenomenon, most people perceive a bowl of fruit. At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and our technology can also manipulate a broad range of wavelengths. Optical fiber transmits light which, although not suitable for direct viewing, can carry data that can be translated into sound or an image. The coding used in such data is similar to that used with radio waves. Ultraviolet lightNext in frequency comes ultraviolet (UV). This is radiation whose wavelength is shorter than the violet end of the visible spectrum. Being very energetic, UV can break chemical bonds, making molecules unusually reactive or ionizing them, in general changing their mutual behavior. Sunburn, for example, is caused by the disruptive effects of UV radiation on skin cells, which can even cause skin cancer, if the radiation damages the complex DNA molecules in the cells (UV radiation is a proven mutagen). The Sun emits a large amount of UV radiation, which could quickly turn Earth into a barren desert, but most of it is absorbed by the atmosphere's ozone layer before reaching the surface. X-raysAfter UV come X-rays. Hard X-rays have shorter wavelengths than soft X-rays. X-rays are used for seeing through some things and not others, as well as for high-energy physics and astronomy. Neutron stars and accretion disks around black holes emit X-rays, which enable us to study them. X-rays will pass through most substances, and this makes them useful in medicine and industry. X-rays are given off by stars, and strongly by some types of nebulae. An X-ray machine works by firing a beam of electrons at a "target". If we fire the electrons with enough energy, X-rays will be produced. Gamma raysAfter hard X-rays come gamma rays. These are the most energetic photons, having no lower limit to their wavelength. They are useful to astronomers in the study of high-energy objects or regions and find a use with physicists thanks to their penetrative ability and their production from radioisotopes. The wavelength of gamma rays can be measured with high accuracy by means of Compton scattering. Note that there are no defined boundaries between the types of electromagnetic radiation. Some wavelengths have a mixture of the properties of two regions of the spectrum. For example, red light resembles infra-red radiation in that it can resonate some chemical bonds. See alsoReferences
|
|||||||||||||||
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electromagnetic_spectrum". A list of authors is available in Wikipedia. | |||||||||||||||





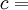 frequency x wavelength or
frequency x wavelength or 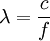 and
and 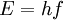 or
or 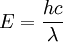
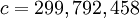 m/s (
m/s (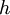 is Planck's constant,
is Planck's constant,