To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
ElectrophoresisFor specific types of electrophoresis (for example, the process of administering medicine, iontophoresis), see electrophoresis (disambiguation). Electrophoresis is the most known electrokinetic phenomena. It was discovered by Reuss in 1809 [1]. He observed that clay particles dispersed in water migrate under influence of an applied electric field. There are detailed descriptions of Electrophoresis in many books on Colloid and Interface Science[2], [3], [4],[5],[6], [7]. There is an IUPAC Technical Report [8] prepared by a group of most known world experts on the electrokinetic phenomena.
Electrophoresis occurs because particles dispersed in a fluid almost always carry an electric surface charge. An electric field exerts electrostatic Coulomb force on the particles through these charges. Another force is electrostatic as well. It is known from double layer theory that all surface charges in fluids are screened with a diffuse layer. This diffuse layer has the same absolute charge value, but with opposite sign from the surface charge. The electric field induces force on the diffuse layer, as well as on the surface charge. The total value of this force equals to the first mentioned force, but it is oppositely directed. However, only part of this force is applied to the particle. It is actually applied to the ions in the diffuse layer. These ions are at some distance from the particle surface. They transfer part of this electrostatic force to the particle surface through viscous stress. This part of the force that is applied to the particle body is called electrophoretic retardation force. There is one more electric force, which is associated with deviation of the double layer from spherical symmetry and surface conductivity due to the excees ions in the diffuse layer. This force is called the electrophoretic relaxation force All these forces are balanced with hydrodynamic friction, which affects all bodies moving in viscous fluids with low Reynolds number. The speed of this motion v is proportional to the electric field strength E if the field is not too strong. Using this assumption makes possible the introduction of electrophoretic mobility μe as coefficient of proprtionality between particle speed and electric field strength: Multiple theories were developed during 20th century for calculating this parameter. Ref. 1 provides an overview. Here are some of the most general conclusions. Product highlight
Theory
The most known and widely used theory of electrophoresis was developed by Smoluchowski in 1903 [9], according to whom the electrophoretic mobility is
where ε is the dielectric constant of the dispersion medium, ε0 is the permittivity of free space (C² N m-2), η is dynamic viscosity of the dispersion medium (Pa s), and ζ is zeta potential (i.e., the electrokinetic potential of the slipping plane in the double layer). Smoluchowski theory is very powerful because it is valid for dispersed particles of any shape and any concentration. However, it has limitations, as it does not include the Debye length κ-1. However, the Debye length must be important for electrophoresis, as follows from the Figure on the right. Increasing the thickness of the DL leads to moving the point of retardation force further from the particle surface. The thicker the DL, the smaller retardation force must be. Detailed theoretical analysis proved that Smoluchowski theory is valid only for a sufficiently thin DL, when the Debye length is much smaller than the particle radius a:
This model of the "thin Double Layer" offers tremendous simplifications not only for electrophoresis theory but for many other electrokinetic theories. This model is valid for most aqueous systems because the Debye length is only a few nanometers there. It breaks only for nano-colloids in a solution with ionic strength close to water. Smoluchowski theory also neglects the contribution of surface conductivity. This is expressed in modern theory as conduction of small Dukhin number
The creation of an electrophoretic theory with a wider range of validity was a purpose of many studies during 20th century. One of the most known considers an opposite asymptotic case when Debye length is larger than particle radius:
It is a "thick Double Layer" model. A corresponding electrophoretic theory was created by Huckel in 1924 [10]. It yields the following equation for electrophoretic mobility:
This model can be useful for some nano-colloids and non-polar fluids, where the Debye length is much larger.
Modern, rigorous theories that are valid for any Zeta potential and often any κa, stem mostly from the Ukrainian (Dukhin, Shilov and others) and Australian (O'Brien, White, Hunter and others) Schools. Historically the first one was Dukhin-Semenikhin theory [13]. Similar theory was created 10 years later by O'Brien and Hunter [14]. Assuming thin Double Layer, these theories would yield results that are very close to the numerical solution provided by O'Brien and White [15]. ApplicationsThere are many applications of electrophoresis for measurements and various operations with particulates Measurementelectrophoresis is used for studying properties of dispersed particles. In particular, for measuring zeta potential. There are several different variations of electrophoresis based techniquies. The most known are: microelectrophoresis and electrophoretic light scattering. These methods are described in details in the "Fundamentals of Interface and Colloid Science", by Lyklema [16] Gel electrophoresisGel electrophoresis is an application of electrophoresis in molecular biology. Biological macromolecules – usually proteins, DNA, or RNA – are loaded on a gel and separated on the basis of their electrophoretic mobility.[17] (The gel greatly retards the mobility of all molecules present.)[18] Electrophoretic displaysElectrophoretic displays (EPD's) are a class of information display that form images by electrophoretic motion of charged, colored pigment particles. Products incorporating electrophoretic displays include the Sony Librie electronic book reader, and the iRex iLiad e-newspaper tablet, both of which use electrophoretic films manufactured by E Ink Corporation. Electrophoretic fingerprintingElectrophoresis is also used in the process of DNA fingerprinting. Certain DNA segments that vary vastly among humans are cut at recognition sites by restriction enzymes (restriction endonuclease). After the resulting DNA fragments are run through electrophoresis, the distance between bands are measured and recorded as the DNA "fingerprint." Electrophoretic depositionCoatings, such as paint or ceramics, can be applied by electrophoretic deposition. The technique can even be used for 3-D printing. References
ISO electrophoresis See also
Categories: Electrophoresis | Colloidal chemistry |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Electrophoresis". A list of authors is available in Wikipedia. |


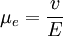



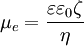 ,
,
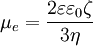 ,
,


