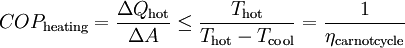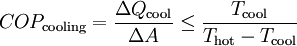To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Heat pumpA heat pump is a machine or device that moves heat from one location (the 'source') to another location (the 'sink' or 'heat sink'), using work. Most heat pump technology moves heat from a low temperature heat source to a higher temperature heat sink.[1] Common examples are food refrigerators and freezers and air conditioners and reversible-cycle heat pumps for providing thermal comfort. Heat pumps can be thought of as an heat engine which is operating in reverse. One common type of heat pump works by exploiting the physical properties of an evaporating and condensing fluid known as a refrigerant. In heating, ventilation, and cooling (HVAC) applications, a heat pump normally refers to a vapor-compression refrigeration device that includes a reversing valve and optimized heat exchangers so that the direction of heat flow may be reversed. Most commonly, heat pumps draw heat from the air or from the ground. Air-source heat pumps do not work well when temperatures fall below around −5°C (23°F). Product highlight
OperationAccording to the second law of thermodynamics heat cannot spontaneously flow from a colder location to a hotter area; work is required to achieve this.[2] Heat pumps differ in how they apply this work to move heat, but they can essentially be thought of as heat engines operating in reverse. A heat engine allows energy to flow from a hot 'source' to a cold heat 'sink', extracting a fraction of it as work in the process. Conversely, a heat pump requires work to move thermal energy from a cold source to a warmer heat sink. Since the heat pump uses a certain amount of work to move the heat, the amount of energy deposited at the hot side is greater than the energy taken from the cold side by an amount equal to the work required. Conversely, for a heat engine, the amount of energy taken from the hot side is greater than the amount of energy deposited in the cold heat sink since some of the heat has been converted to work. One common type of heat pump works by exploiting the physical properties of an evaporating and condensing fluid known as a refrigerant. The working fluid, in its gaseous state, is pressurized and circulated through the system by a compressor. On the discharge side of the compressor, the now hot and highly pressurized gas is cooled in a heat exchanger called a condenser until it condenses into a high pressure, moderate temperature liquid. The condensed refrigerant then passes through a pressure-lowering device like an expansion valve, capillary tube, or possibly a work-extracting device such as a turbine. This device then passes the low pressure, barely liquid (saturated vapor) refrigerant to another heat exchanger, the evaporator where the refrigerant evaporates into a gas via heat absorption. The refrigerant then returns to the compressor and the cycle is repeated. In such a system it is essential that the refrigerant reaches a sufficiently high temperature when compressed, since the second law of thermodynamics prevents heat from flowing from a cold fluid to a hot heat sink. Similarly, the fluid must reach a sufficiently low temperature when allowed to expand, or heat cannot flow from the cold region into the fluid. In particular, the pressure difference must be great enough for the fluid to condense at the hot side and still evaporate in the lower pressure region at the cold side. The greater the temperature difference, the greater the required pressure difference, and consequently more energy is needed to compress the fluid. Thus as with all heat pumps, the energy efficiency (amount of heat moved per unit of input work required) decreases with increasing temperature difference. Thus a ground-source heat pump, which has a very small temperature differential, is relatively efficient. (Figures of 75% and above are quoted.) Due to the variations required in temperatures and pressures, many different refrigerants are available. Refrigerators, air conditioners, and some heating systems are common applications that use this technology. In HVAC applications, a heat pump normally refers to a vapor-compression refrigeration device that includes a reversing valve and optimized heat exchangers so that the direction of heat flow may be reversed. The reversing valve switches the direction of refrigerant through the cycle and therefore the heat pump may deliver either heating or cooling to a building. In the cooler climates the default setting of the reversing valve is heating. The default setting in warmer climates is cooling. Because the two heat exchangers, the condenser and evaporator, must swap functions, they are optimized to perform adequately in both modes. As such, the efficiency of a reversible heat pump is typically slightly less than two separately-optimized machines. In plumbing applications, a heat pump is sometimes used to heat or preheat water for swimming pools or domestic water heaters. In somewhat rare applications, both the heat extraction and addition capabilities of a single heat pump can be useful, and typically results in very effective use of the input energy. For example, when an air cooling need can be matched to a water heating load, a single heat pump can serve two useful purposes. Unfortunately, these situations are rare because the demand profiles for heating and cooling are often significantly different. RefrigerantsUntil the 1990s, the common refrigerant were often chlorofluorocarbons such as R-12 (dichlorodifluoromethane), one in a class of several refrigerants using the brand name Freon, a trademark of DuPont. Its manufacture was discontinued in 1995 because of the damage that CFCs cause to the ozone layer if released into the atmosphere. One widely-adopted replacement refrigerant is the hydrofluorocarbon (HFC) known as R-134a (1,1,1,2-tetrafluoroethane). Interestingly, R-134a is not as efficient as the R-12 it replaced (in automotive applications) and therefore, more energy is required to operate systems utilizing R-134a than those using R-12. Other substances such as liquid ammonia, or occasionally the less corrosive but flammable propane or butane, can also be used. Since 2001, carbon dioxide, R-744, has increasingly been used, utilizing the transcritical cycle. In residential and commercial applications, the hydrochlorofluorocarbon (HCFC) R-22 is still widely used, however, HFC R-410a is considered to be more environmentally friendly, and thus is increasingly being used. Hydrogen, Helium, Nitrogen, or plain air is used in the Stirling cycle, providing the maximum number of options in environmentally friendly gases. EfficiencyWhen comparing the performance of heat pumps, it is best to avoid the word "efficiency" which has a very specific thermodynamic definition. The term coefficient of performance (COP) is used to describe the ratio of useful heat movement to work input. Most vapor-compression heat pumps utilize electrically powered motors for their work input. However, in most vehicle applications shaft work, via their internal combustion engines, provide the needed work. When used for heating a building on a mild day, a typical heat pump has a COP of three to four, whereas a typical electric resistance heater has a COP of 1.0. That is, one joule of electrical energy will cause a resistance heater to produce one joule of useful heat, while under ideal conditions, one joule of electrical energy can cause a heat pump to move much more than one joule of heat from a cooler place to a warmer place. Sometimes this is inappropriately expressed as an efficiency value greater than 100%, as in the statement, "XYZ brand heat pumps operate at up to 400% efficiency!" This is inaccurate, since the work does not make heat, but instead moves existing heat "upstream"; otherwise, this would be a perpetual-motion machine. Note that when there is a wide temperature differential, e.g., when heating a house on a very cold winter day, it takes more work to move the same amount of heat indoors as on a mild day. Ultimately, due to Carnot efficiency limits, the heat pump's performance will approach 1.0 as the outdoor-to-indoor temperature difference increases. This typically occurs around −18 °C (0 °F) outdoor temperature for air-source heat pumps (ground source heat pumps are dependent upon the temperature underground). Also, as the heat pump takes heat out of the air, some moisture in the outdoor air may condense and possibly freeze on the outdoor heat exchanger. The system must periodically melt this ice. In other words, when it is extremely cold outside, it is simpler, and wears the machine less, to heat using an electric-resistance heater than to strain an air-coupled heat pump. In cooling mode a heat pump's operating performance is described as its energy efficiency ratio (EER) or seasonal energy efficiency ratio (SEER), and both measures have units of BTU/(h·W). A larger EER number indicates better performance. The manufacturer's literature should provide both a COP to describe performance in heating mode and an EER or SEER to describe performance in cooling mode. Actual performance varies, however, and depends on many factors such as installation, temperature differences, site elevation, and maintenance. Heat pumps are more effective for heating than for cooling if the temperature difference is held equal. This is because the compressor's input energy is largely converted to useful heat when in heating mode, and is discharged along with the moved heat via the condenser. But for cooling, the condenser is normally outdoors, and the compressor's dissipated work is rejected rather than put to a useful purpose. For the same reason, opening a food refrigerator or freezer heats up the kitchen rather than cooling it because its refrigeration cycle rejects heat to the indoor air. This heat includes the compressor's dissipated work as well as the heat removed from the inside of the appliance. The COP for a heat pump in a heating or cooling application, with steady-state operation, is:
Heat sourcesA number of sources have been used for the heat source for heating buildings. Most commonly, heat pumps draw heat from the air or from the ground. The heat drawn from the ground is in most cases stored solar heat, and it should not be confused with geothermal heat, though the latter will contribute in some small measure to all heat in the ground. Other heat sources include water; nearby streams and other natural water bodies have been used, and sometimes domestic waste water which is often warmer than the ambient temperature. The technologies are developing rapidly: COPs (coefficient of performance) have risen from COP=3 to COP=4 or even COP=5 over the last five years. Heat pumps are now becoming popular choices for home-heating as well as for cooling — especially in areas with less severe winters. Those buying air-source heat pumps should look closely at its COP, the outside temperature range in which that COP is effective, the cost of installation, how much heat it can move, and how much noise it generates. Air-source heat pumps do not work well when temperatures fall below around −5°C (23°F). Ground-source heat pumps typically have higher COPs than air-coupled heat pumps, because they draw heat from ground or groundwater, and this is at a relatively constant temperature all year-round below a depth of about eight feet (2.5 m). The tradeoff for this improved performance is that a ground-coupled heat pump is usually more complicated due to the need for wells or buried coils, and thus is also usually much more expensive to install than an air-coupled heat pump. Solid State Heat PumpsIn 1881, the German physicist Emil Warburg put a block of iron into a strong magnetic field and found that it increased very slightly in temperature. Some commercial ventures to implement this technology are underway, claiming to cut energy consumption by 40% compared to current domestic refrigerators.[3] The process works as follows: Powdered gadolinium is moved into a magnetic field, heating the material by 2 to 5 °C. The heat is removed by a circulating fluid. The material is then moved out of the magnetic field, reducing its temperature below its starting temperature. See also
References |
|
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Heat_pump". A list of authors is available in Wikipedia. |