Tiny test tube experiment shows reaction of melting materials at the nano scale
Advertisement
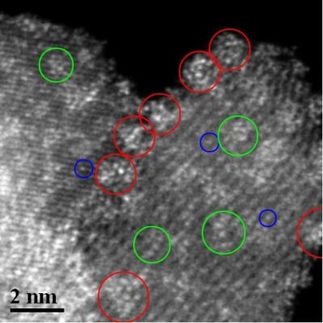
Researchers at The University of Texas at Austin have conducted a basic chemistry experiment in what is perhaps the world's smallest test tube, measuring a thousandth the diameter of a human hair.
The nano-scale test tube is so small that a high-power electron microscope was required to see the experiment.
Made from a thin shell of carbon, the test tube was stuffed with a thread-like crystal (a nanowire) of germanium with a tiny particle of gold at its tip.
The researchers heated the test tube and watched as the gold melted at the end of the nanowire, much like any solid crystal heated above its melting temperature in a glass test tube.
"The experiment is relatively simple," said chemical engineer Brian Korgel, whose laboratory conducted it. "Essentially, we observe well-known phenomena, like melting, capillarity and diffusion, but at a much, much smaller scale than has been possible to see before."
Such experiments provide new fundamental insights about how nanomaterials behave, and might be used to create new technologies, from better solar cells to unprecedentedly strong yet light-weight materials to higher performance optical displays and computing technologies.
Korgel and graduate students Vincent Holmberg and Matthew Panthani conducted the experiment, which was reported in the Oct. 16 edition of Science.
During the experiment, the nanowire melted as the temperature rose, but its shape was retained because the carbon test tube maintained its shape.
"In these very small structures, the phase behavior (like its melting temperature, etc.) can be different than bulk materials and can be size-dependent," Korgel said. "Therefore, if the structure changes when the phase change happens, then the result becomes very difficult to interpret and in fact, may not even represent the true behavior of the system."
The carbon test tube, however, provided a rigid container for studying what happens when materials are heated and melted at the nanoscale.
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.