Polarized light: A simple route to highly chiral materials
Advertisement
chirality is at the heart of chemical research and much technology. For organic chemists, choosing between the left- and right-handed isomers of molecules is all part of a day's work. However, many solid materials also have enantiomeric forms, giving rise to a range of applications.
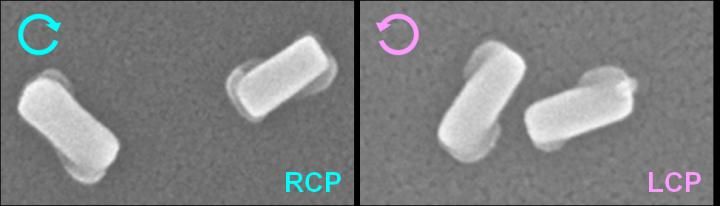
Nanostructures prepared by RCP and LCP light irradiation.
2018 Tetsu Tatsuma, Institute of Industrial Science, The University of Tokyo
Organic chemists generally rely on an arsenal of laboratory reactions to control chiral purity. For materials, there is another, more elegant approach - circularly polarized light, which is readily made, and can be either left-circularly polarized (LCP) or right-circularly polarized (RCP). In material synthesis, the opposite twists of LCP and RCP light indirectly lead to structures that are mirror images of each other.
Previously, this strategy has been hampered in practice. Now, researchers at The University of Tokyo's Institute of Industrial Science have successfully created chiral nanostructures from particles of gold (Au). The trick was to use circularly polarized light to generate electric fields, which localize differently depending on LCP or RCP. This in turn drove the chiral deposition of a dielectric material.
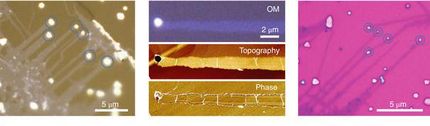
As described in a study the researchers first deposited Au nanocuboids - essentially miniature rectangular gold bars - on a TiO2 substrate.
As study co-author Koichiro Saito explains, "Under a beam of circularly polarized light, electric fields built up around the cuboids - but at one pair of corners for LCP rotation, and the opposite pair under RCP light. At this point, we had achieved chirality, but in electric rather than material form."
The chirality of the electric field was then transferred to the material itself by plasmon-induced charge separation, in which Pb2+ ions were oxidized through the chirally distributed electric fields. This deposited PbO2, a dielectric material, at either one set of cuboid corners or the other, depending on the original light source. Electron microscopy showed the gold bars transformed into non-superimposable mirror images, the hallmark of chirality.
"This is the first time a chiral material has been made by exploiting plasmon resonance," co-author Tetsu Tatsuma says. "No other source of chirality is needed but light itself. Nanoscale chiral plasmonic materials are highly useful for sensing and asymmetric synthesis, and our process makes them much more efficient to produce. Plus, we don't think it's limited to one product - other chiral nanomaterials have an incredible range of functions in modern technology."
Original publication
Koichiro Saito and Tetsu Tatsuma; "Chiral Plasmonic Nanostructures Fabricated by Circularly Polarized Light"; Nano Letters; 2018
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.