Self-organizing molecules: Cups with attoliter volume
Advertisement
They look like interlocking egg cups, but a hen's egg is 100,000 times as thick as one of the miniature cups: Scientists at the Center for Nanointegration (CENIDE) at the University of Duisburg-Essen (UDE) have made polymers to form themselves into tiny cups on their own. They could, for example, be used to remove oil residues from water.
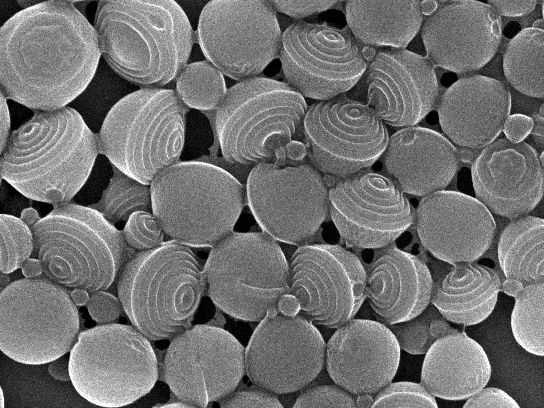
Stacked Janus nanocups, before being separated.
(c) UDE, AG Gröschel
The first step is a very thin long chain of so-called triblock terpolymers – a kind of plastic. If many of them are added to a droplet of a solvent, they automatically adapt their shape to the round boundary. When the solvent evaporates the drop becomes smaller and the polymer cup structure shrinks with it. What remains are the cups, which, like Russian Matryoshka dolls, lie one inside the other. Xiaolian Qiang, a PhD candidate in the research group of junior professor André Gröschel, then separates them in several steps.
"Such particles are called ‘Janus particles’ in chemistry because they have two sides with different properties", the 29-year-old explains. "The term comes from the Roman god Janus, who had two faces." Everyone knows the particle’s opposite chemical properties from soap: One half is hydrophilic, i.e. it dissolves well in water. The other half is hydrophobic like a drop of oil and therefore insoluble in water. In Qiang's experiments, 50 percent of cups are produced with hydrophilic inner and hydrophobic outer sides. The other half is the other way round.
Qiang utilizes this principle to fill the small vessels with nanoparticles, for example. If she surrounds the particles with a solvent they do not like, they flee into the interior of the cup with the correspondingly opposite property. In this way it is possible to absorb oil residues from water or to transport substances through an aqueous environment – e.g. blood.
Now, Qiang and her team work on creating only one of the two variants.