Old newspapers can be used to grow carbon nanotubes
Advertisement
A research collaboration between Rice University and the Energy Safety Research Institute (ESRI) at Swansea University has found that old newspapers can be used as a low cost, eco-friendly material on which to grow single walled carbon nanotubes on a large scale.

Research shows old newspapers can be used to grow carbon nanotubes (symbolic image).
Pexels, pixabay.com, CC0
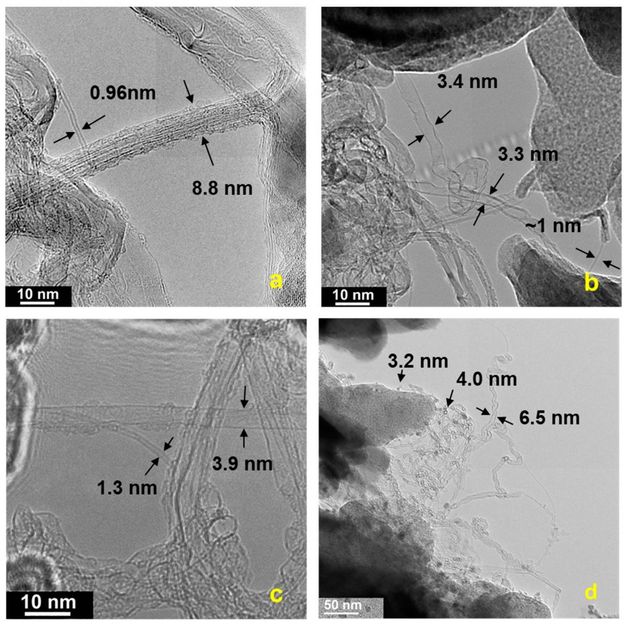
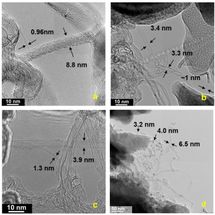
TEM images of raw carbon soot grown on kaolin sized paper showing (a) roped single-walled carbon nanotubes (SWCNTs) helically wrapped by a SWCNT, and large SWCNTs, (b) collapsed, (c) folded, and (d) twisted nanotubes. Scale bar = 10 nm (a-c) and 50 nm (d).
Rice University

Carbon nanotubes are tiny molecules with incredible physical properties that can be used in a huge range of things, such as conductive films for touchscreen displays, flexible electronics, fabrics that create energy and antennas for 5G networks.
The new study details the research experiments carried out in producing carbon nanotubes which could have the potential to solve some of the problems associated with their large scale production such as:
- The high cost of preparing a suitable surface for chemical growth.
- The difficulties in scaling up the process, as only single surface growth processes have been previously available.
The research team discovered that the large surface area of newspapers provided an unlikely but ideal way to chemically grow carbon nanotubes.
Lead researcher Bruce Brinson said: "Newspapers have the benefit of being used in a roll-to-roll process in a stacked form making it an ideal candidate as a low-cost stackable 2D surface to grow carbon nanotubes."
However, not all newspaper is equally good - only newspaper produced with sizing made from kaolin, which is china clay, resulted in carbon nanotube growth.
Co-author Varun Shenoy Gangoli said: "Many substances including talc, calcium carbonate, and titanium dioxide can be used in sizing in papers which act as a filler to help with their levels of absorption and wear. However it was our observation that kaolin sizing, and not calcium carbonate sizing, showed us how the growth catalyst, which in our case was iron, is affected by the chemical nature of the substrate."
ESRI Director Andrew Barron, also a professor at Rice University in the USA, said: "While there have been previous research that shows that graphene, carbon nanotubes and carbon dots can be been synthesised on a variety of materials, such as food waste, vegetation waste, animal, bird or insect waste and chemically grown on natural materials, to date, this research has been limited.
"With our new research, we have found a continuous flow system that dramatically reduces the cost of both substrate and post synthesis process which could impact on the future mass manufacture of single walled carbon nanotubes."