Scientists probe electronic angular momentum to a chemical reaction for the first time
Advertisement
A chemical reaction can be understood in detail at the quantum state-resolved level, through a combined study of molecular crossed beam experiments and theoretical quantum molecular reaction dynamics simulations.
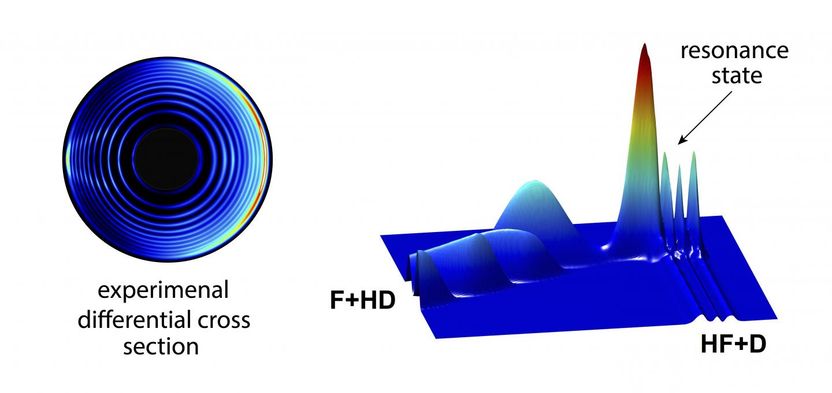
he left circles are the experimental measurement of the product state-resolved differential cross sections of the F+HD reaction, the right image is the related partial-wave resonance wavefunction of the reaction.
DICP
At a single collision condition, the molecular crossed beam apparatus is able to detect the scattering angle-resolved product with rotational state-resolution. Whereas, with accurate global potential energy surface, quantum reactive scattering theory is able to predict the corresponding reactive scattering information.
In previous studies, the chemical reaction dynamics was revealed only with the product rotational state-resolution. And the investigation of a reaction at a finer level would be an inspiring break through.
Recently, Professor YANG Xueming from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and Professor WANG Xing'an from the University of Science and Technology of China developed molecular crossed beam apparatus with threshold ionization velocity map imaging technique, enabling to probe the scattering product with high angular resolution with quantum rotational-state recognition.
With this powerful apparatus, in combined with new quantum reactive scattering theory developed by Professor SUN Zhigang from the DICP, which included the electronic angular momentum effect, the electronic angular momentum effect to a chemical reaction was revealed for the first time.
This finding was published online in Science.
There is distinguished reactive scattering quantum resonance in the F + HD (the Fluorine atom with the HD isotope of the H2 molecule) reaction. It has been taken as the prototype to resolve partial wave resonance structures in a chemical reaction.
With this feature, the scientists thought that the role of the electronic angular momentum of the F atom in this chemical reaction would be recognized. The F atom was characterized by p electronic orbit with l=1, which could influence the partial wave resonance structures.
It was found that, by including the electronic angular momentum, the single partial wave structure would split into four-fold partial wave resonance structure, which was capable of varying the angular distributions of the chemical product.
The energy of the electronic angular momentum is much smaller than the rotational energy of a diatomic molecule (~ several tens wave number). Its influence to a chemical reaction is subtle and difficult to detect.

































































