New substance classes for nanomaterials
Nano spheres and diamond slivers made of silicon and germanium
Advertisement
Chemists at Goethe University Frankfurt have developed two new classes of materials in the field of nanomaterials and investigated them together with their cooperation partners at the University of Bonn: for the first time, they have succeeded in producing a nano sphere of silicon atoms and a building block for a diamond-like crystal of the semiconductor elements silicon and germanium. The two new classes of materials have potential applications in the miniaturisation of computer chips, in high-resolution screens for smartphones, and in solar cells and light-emitting diodes with the highest levels of efficiency.
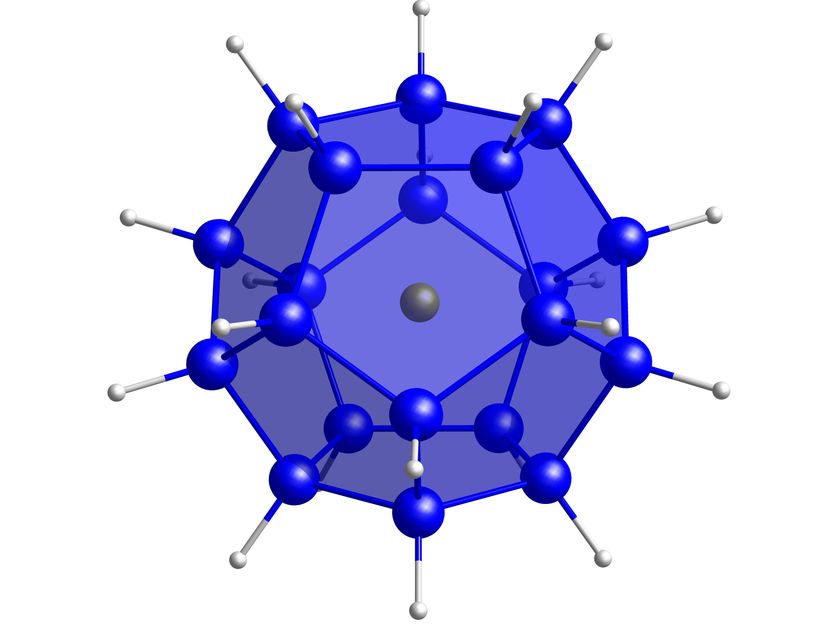
The silicon sphere [Cl@Si20H20]−, synthesised for the first time by chemists from Goethe University Frankfurt, promises new applications in semiconductor technology. Blue: silicon, gre chloride ion, grey: hydrogen.
Goethe University Frankfurt
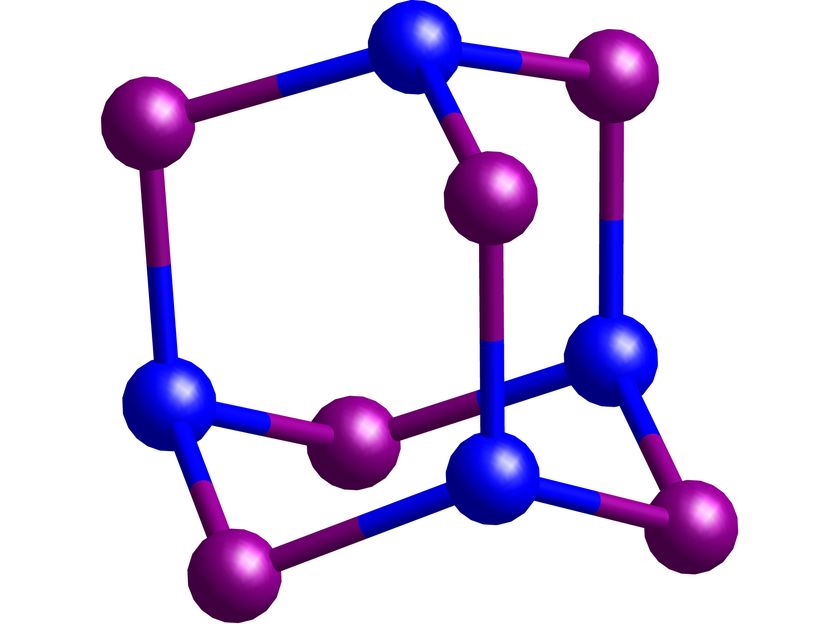
Building block for silicon-germanium alloys: A section of the silicon-germanium adamantane synthesised in Frankfurt (shown here without substituents). Blue: silicon, magenta: germanium.
Goethe University Frankfurt
The latest generations of computer chips are only a few nanometres in size and are becoming ever more energy-saving and powerful as a result of progressive miniaturisation. Since the etching processes traditionally used in chip production are increasingly reaching their limits, the development of new, nanostructured semiconductor materials is essential. Such nano semiconductors also play a central role in converting electricity into light and vice versa.
A team at Goethe University Frankfurt led by Matthias Wagner has now succeeded in synthesising molecular nano "spheres" made of 20 silicon atoms, so-called silafulleranes. The second new class of materials are crystal building blocks made of 10 silicon and germanium atoms that have a diamond-like structure. Decisive insights into the electronic structures of the new compounds were provided by computer-based theoretical analyses from Stefan Grimme's research group in Bonn.
The 20 silicon atoms of silafullerane form a dodecahedron, a body composed of regular pentagons. It encapsulates a chloride ion. A hydrogen atom protrudes outward at each silicon corner of the body. Doctoral student Marcel Bamberg, who synthesised the molecule, explains: "Our silafullerane is the long-sought progenitor of this new class of substances. The hydrogen atoms can easily be replaced with functional groups, thus giving the silafullerane different properties." Bonn quantum chemist Markus Bursch adds: "We support the targeted generation of potentially useful properties with theoretical predictions of their resulting effects."
The silicon-germanium adamantane represents the building block of a mixed silicon-germanium alloy. Benedikt Köstler, who is developing the compounds as part of his doctoral thesis, says: "Recent studies have shown that silicon-germanium alloys are superior to pure silicon semiconductors in important application areas. However, the production of such alloys is very difficult and you often get mixtures of different compositions. We have succeeded in developing a simple synthesis path for the basic building block of silicon-germanium alloys. Our silicon-germanium adamantane therefore enables the investigation of important chemical and physical properties of silicon-germanium alloys on the molecular model. We also want to use it in the future to produce silicon-germanium alloys with faultless crystal structures."
Carbon, which is chemically very similar to the elements silicon and germanium, occurs in comparable forms to the two new classes of substances: Hollow spheres of carbon atoms ("fullerenes") correspond to silafulleranes, and diamonds consisting of carbon are composed of adamantane subunits. Among other things, fullerenes increase the efficiency of organic solar cells, could make the batteries of electric cars safer, and promise progress in high-temperature superconductivity. Nanodiamonds also have a wide range of applications, from pharmaceuticals to catalysis research.
Against this background, the researchers in Frankfurt and Bonn are excited to see in which fields their silafulleranes and silicon-germanium adamantanes will become established. Matthias Wagner says: "It is already possible to generate light in all colours of the visible spectrum with nanostructured silicon and germanium in the form of quantum dots, and this is being tested for computer and mobile phone displays, as well as in telecommunications. Apart from the chemical-technical potential, I am personally fascinated by the high symmetry of our compounds: For example, our silafullerane is one of the five Platonic solids and possesses a timeless beauty."
Original publication
(1) Marcel Bamberg, Markus Bursch, Andreas Hansen, Matthias Brandl, Gabriele Sentis, Lukas Kunze, Michael Bolte, Hans-Wolfram Lerner, Stefan Grimme, Matthias Wagner; "[Cl@Si20H20]−: Parent Siladodecahedrane with Endohedral Chloride Ion"; J. Am. Chem. Soc. 2021, 143, 10865–10871.
(2) Benedikt Köstler, Michael Bolte, Hans-Wolfram Lerner, Matthias Wagner; "Selective One-Pot Syntheses of Mixed Silicon-Germanium Heteroadamantane Clusters"; Chem. Eur. J.






























































