Kekulé's shattered dream: snakes become ladders
Researchers create novel molecules that serve as ziplines for energy
Advertisement
Researchers from the Universities of Bonn and Regensburg move packets of energy along a molecular ladder made of hundreds of benzene rings. Such polymers can potentially be used to design new displays based on organic light-emitting diodes, or for solar cells. The extraordinary material is now described in the journal Nature Communications.
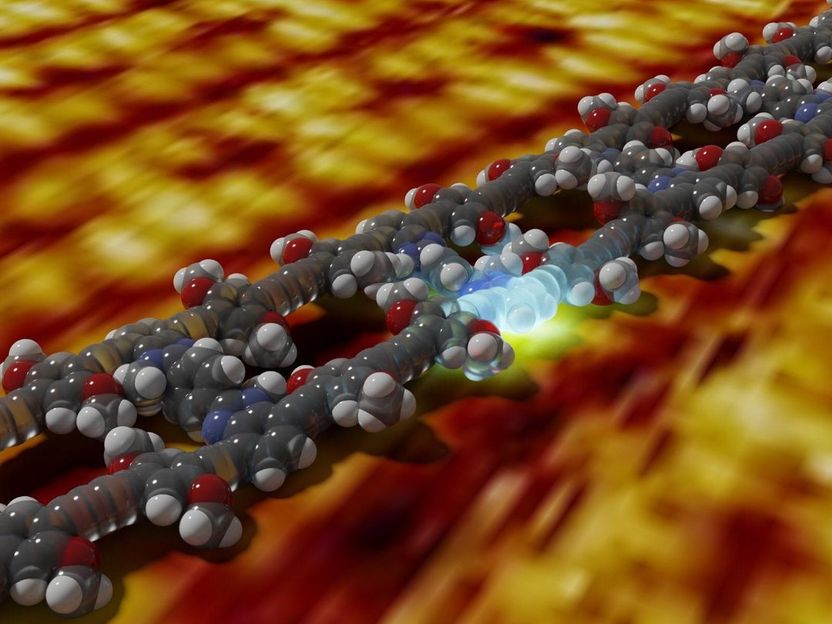
A packet of energy is generated on a molecular ladder by the absorption of light. The background image shows a real measurement of the ladder structure with a scanning tunneling microscope.
© Joshua Bahr und Tristan Keller
In the 19th century, the scientific community puzzled over how the atoms in the mysterious compound benzene were arranged. This "aromatic" molecule soon proved to have a surprisingly simple structure: It consisted of six carbon and six hydrogen atoms. But how could these twelve atoms arrange themselves in space to form a chemically stable object? The chemist Friedrich August Kekulé, later professor at the University of Bonn, brought light into the darkness. Legend has it that he sat dozing by the fireplace in the winter of 1861. Kekulé suddenly had a vision of a snake devouring its own tail. He realized that the carbon atoms of benzene must be organized in a circle, similar to a small wagon wheel.
"This dream ultimately laid the foundation for the massive expansion of the chemical industry toward the end of the 19th century," says Prof. Sigurd Höger of the Kekulé Institute of Organic Chemistry and Biochemistry at the University of Bonn, who is a member of the Transdisciplinary Research Area "Building Blocks of Matter and Fundamental Interactions" at the University of Bonn. Benzene is an important building block for paints, pharmaceuticals and plastics, for example.
Hundreds of benzene rings in the shape of a ladder
Although the wheel is often touted as mankind’s oldest invention, the ladder is actually quite a bit older. Kekulé's successors at the University of Bonn had long been dreaming of molecules in the shape of a ladder, consisting of hundreds of benzene rings. The researchers from the Kekulé Institute and the Mulliken Center for Theoretical Chemistry at the University of Bonn, together with a team led by Prof. John Lupton from the Institute of Experimental and Applied Physics at the University of Regensburg, have now constructed such a molecular ladder. This is a molecule with two tracks of so-called "conjugated polymers", in which double and single bonds alternate between the carbon atoms. They make up the rails that you hold on to when climbing up an ordinary ladder.
The researchers first designed a precursor compound that contained only a single polymer chain and attached polymerizable groups - a flexible "snake." For some of the material, the second rail of the ladder was then formed in a subsequent step by means of a zipper reaction, much like when close an anorak. In this way, in addition to the polymer with a single conjugated rail, the team obtained a polymer with two conjugated rails - the stiff "ladder". Both polymers were of equal length and could now be compared to each other: How would turning a snake into a ladder affect the material’s properties?
The researchers examined the structure using a scanning tunneling microscope. The tiny molecular ladder is one nanometer (a millionth of a millimeter) high, two nanometers wide and one hundred nanometers long. The chemists also confirmed the shape and extraordinary rigidity of the ladders - compared to the snakes - through extensive computer simulations using a novel theory that predicts the individual motions of all atoms within the molecule.
Potential building block for electronics
"The ladder structure is retained not only when the molecules are placed on a surface, but also when they are dissolved in a liquid," says Prof. Lupton of the University of Regensburg. This feature, he adds, allows energy to move along the molecule in space, providing a potential building block for optical networks, circuits and sensors.
In principle, such polymers conduct electrical currents and can be used to make new displays based on organic light-emitting diodes (OLEDs), or to convert light into electricity in a solar cell. When light falls onto such a molecule, it is absorbed and produces a small packet of energy. The researchers were able to observe how these packages moved along the ladder virtually unimpeded, as if on a zipline. The open snake-like polymers, on the other hand, do not show this effect. Their properties are similar to those of conventional polymer molecules: the packages slide along the "snakes" and lose energy.
Kekulé's shattered dream
"While old Kekulé 'saw' the single molecule as a ring, he certainly never dreamed that there would one day be giant molecules of such rigidity that they are unable to bite their own tails," says Höger, summarizing the result with a wink.