Tip tricks control reactions in a single molecule
Pulses from an atom-sharp tip enable researchers to break and form chemical bonds at will
Advertisement
chemical reactions often produce messy mixtures of different products. Hence, chemists spend a lot of time coaxing their reactions to be more selective to make particular target molecules. Now, an international team of researchers has achieved that kind of selectivity by delivering voltage pulses to a single molecule through an incredibly sharp tip.
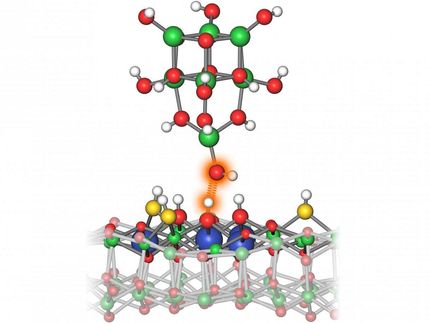
An international team of scientists has developed a method to break and form molecular bonds by applying voltage to a molecule using a sharp tip only a few atoms wide.
© 2022 KAUST; Anastasia Serin
“Controlling the pathway of a chemical reaction, depending on the voltage pulses used, is unprecedented and very alluring to chemists,” says KAUST's Shadi Fatayer.
The team used an instrument that combines scanning tunneling microscopy (STM) and atomic force microscopy (AFM). Both techniques can map out the positions of atoms within individual molecules using a tip that may be just a few atoms wide. But the voltage can also be used to break bonds within a molecule, potentially allowing new bonds to form.
“Tip-controlled reactions have been previously performed, but there was no control over the final product,” Fatayer says. “The selectivity is the key element here — depending on the polarity and value of the voltage pulses, we can form and break different internal bonds at will.”
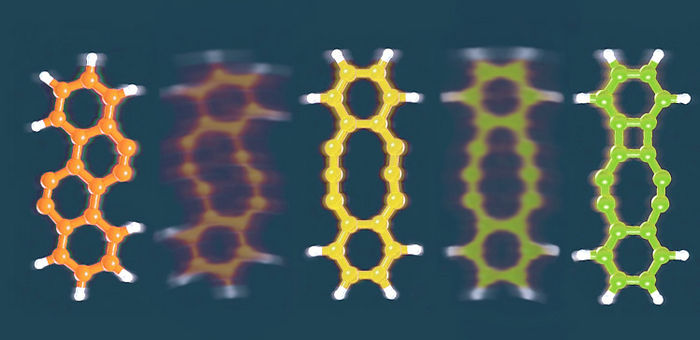
The researchers used this approach to study tetrachlorotetracene, a molecule that contains four chlorine atoms attached to a row of four hexagonal rings of carbon atoms. Applying a voltage of about 3.5V removed two chlorine atoms and prompted the molecule to rearrange. Increasing the voltage removed the remaining chlorine atoms, triggering further rearrangements that formed three different products.
The first product has four hexagonal rings arranged in a zig-zag pattern; the second has a 10-membered ring flanked by two six-membered rings; and the third contains a four-membered ring, an eight-membered ring, and two six-membered rings.
Small voltage pulses could be used to interconvert these products. By fine-tuning the voltage, the researchers could control which bonds were broken and which rearrangement product formed.
Combining their results with theoretical calculations, the researchers showed that the method’s selectivity depends on the landscape of energy states the molecules adopt when they carry different electrical charges, known as their oxidation state. Since the initial oxidation state of a molecule can be controlled by an electric field, this approach could help chemists to design new chemical reactions and products, Fatayer says.
His group is now developing ways to add or remove single electrons to individual molecules, and to apply voltage pulses to specific parts of a molecule to control which chemical reaction occurs.