Enhancement of Li+ transport through intermediate phase in high-content inorganic composite quasi-solid-state electrolytes
A high-proportion inorganic composite quasi-solid-state electrolyte was fabricated through the integration of high-speed defoamed mixers with in situ polymerization methodology.
Advertisement
Quasi-solid-state electrolytes promise the safety of ceramics, the flexibility of polymers, and the conductivity of liquids—yet the “how” behind their superior ion transport has remained murky. Now, a joint team from Fudan University and the National Institute for Cryogenic & Isotopic Technologies (Romania), led by Professors Aishui Yu and Tao Huang, delivers a decisive answer in Nano-Micro Letters. Their review, “Enhancement of Li⁺ Transport Through Intermediate Phase in High-Content Inorganic Composite electrolytes,” decodes the hidden chemistry that lets lithium sprint across solid/liquid boundaries.
A high-proportion inorganic composite quasi-solid-state electrolyte was fabricated through the integration of high-speed defoamed mixers with in situ polymerization methodology.
The Secret Sauce: Acidic Interfaces
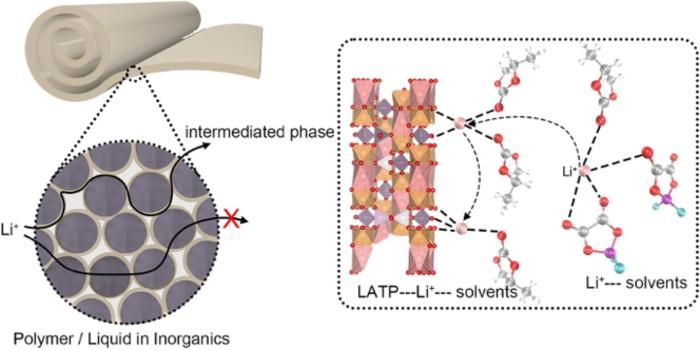
- Selective Anion Anchoring: Acidic LATP surfaces act as Lewis-acid traps for DFOB⁻ anions, loosening Li⁺ solvation cages and jacking the Li⁺ transference number from 0.31 → 0.53.
- Size Matters: Shrinking LATP particles to 200–300 nm boosts specific surface area and pushes ionic conductivity to 0.51 mS cm-1 at room temperature.
- Dual-Phase Highways: An “intermediate phase” bridges ceramic and liquid domains, creating 3-D conduction networks that outpace single-phase polymers.
Performance that Speaks Louder than Theory
- 6000 h non-stop cycling in Li||Li symmetric cells at 0.1 mA cm-2—no short-circuits.
- 80.5 % capacity retention after 200 cycles in a 5 V-class LNMO||Li full cell at 0.5 C.
- Pouch-cell demo drives LED arrays and mini-motors, proving scalability beyond coin cells.
Design Rules for Tomorrow’s Electrolytes
- Surface Engineering > Bulk Chemistry: Acidic surface sites are the true catalysts; neutral or basic variants lag by 30 %.
- Active Fillers Win: Ion-conductive LATP beats inert alumina, cutting activation energy and sustaining high-rate capability (155 mAh g-1 at 0.1 C vs. 82 mAh g-1).
- SEI Self-Defense: LATP-induced decomposition forms a LiF-rich interphase that stops further Ti4+ reduction—self-limiting protection without extra coatings.
Future Outlook
- Nano-Architected Interfaces: Next-gen electrolytes will leverage tunable surface acidity and hierarchical porosity to push conductivities beyond 1 mS cm-1.
- High-Loading Cathodes: 14 mg cm-2 LNMO cathodes already retain 142 mAh g-1—roadmap to >300 Wh kg-1 pouch cells.
- Universal Design Toolkit: The acid-base descriptor framework can be ported to sulfides, chlorides, and beyond, fast-tracking the commercial leap from lab to EV.
Stay tuned as the Yu–Huang team turns interfacial chemistry into the next performance revolution for lithium-metal batteries.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.
































































