Pollution cleanup method destroys toxic “forever chemicals”
Breakthrough process was developed for drinking water treatment and toxic site remediation
Advertisement
An insidious category of carcinogenic pollutants known as “forever chemicals” may not be so permanent after all.

Symbolbild
Unsplash
University of California, Riverside, chemical engineering and environmental scientists recently published new methods to chemically break up these harmful substances found in drinking water into smaller compounds that are essentially harmless.
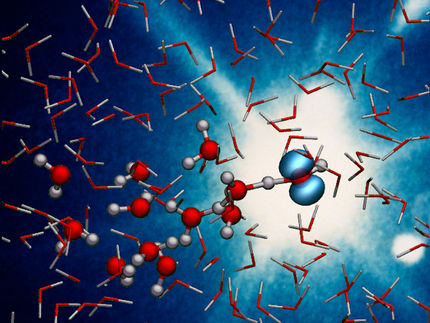
The patent-pending process infuses contaminated water with hydrogen, then blasts the water with high-energy, short-wavelength ultraviolet light. The hydrogen polarizes water molecules to make them more reactive, while the light catalyzes chemical reactions that destroy the pollutants, known as PFAS or poly- and per-fluoroalkyl substances.
This one-two punch breaks the strong fluorine-to-carbon chemicals bonds that make these pollutants so persistent and accumulative in the environment. In fact, the molecular destruction of PFAS increased from 10% to nearly 100% when compared to other ultraviolet water-treatment methods, while no other undesirable byproducts or impurities are generated, the UCR scientists reported in a paper recently published in the Journal of Hazardous Materials Letters.
What’s more, the cleanup technology is green.
“After the interaction, hydrogen will become water. The advantage of this technology is that it is very sustainable,” said Haizhou Liu, an associate professor in UCR’s Department of Chemical and Environmental Engineering and the corresponding author of the paper.
Liu’s laboratory developed the technology with help from a $400,000 grant from the National Science Foundation.
PFAS are a family of thousands of chemical compounds characterized by fully fluorinated carbon atoms with stubbornly strong chemical bonds that last indefinitely in the environment – hence the moniker “forever chemicals.” Examples of PFAS-containing products include grease-resistant paper wrappers and containers such as microwave popcorn bags, pizza boxes, and candy wrappers. They are also found in stain and water repellents used on carpets, upholstery, clothing; cleaning products; non-stick cookware; and paints, varnishes, and sealants, according to the U.S. Environmental Protection Agency.
Studies have linked exposure to certain levels of PFAS to many ill health effects, including increased risk for prostate, kidney, and testicular cancers, as well as decreased fertility or increased high blood pressure in pregnant women, developmental effects or delays in children, low birth weight, and accelerated puberty, according to the EPA.