Diapers reloaded: 200 times faster recycling with light - Recycled plastic molecules can be used in many ways
Water and UV radiation dissolve crosslinked polymers of diaper inserts quickly, energy-efficiently and without chemicals
Advertisement
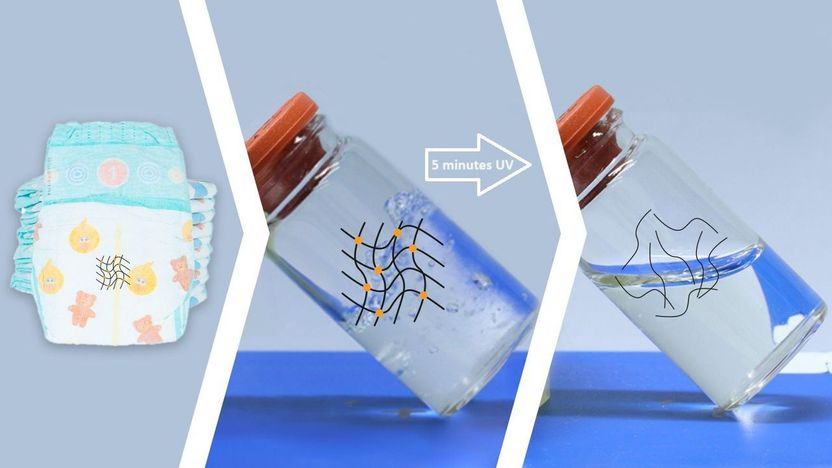
More than 100,000 tons of disposable diapers are thrown away in Germany every year. This means that huge quantities of valuable raw materials end up in the trash, including the absorbent pads. Their core consists of special polymers called superabsorbents. Researchers at the Karlsruhe Institute of Technology (KIT) have now significantly improved the complex recycling of these superabsorbents. With the help of UV radiation, they dissolve the chemical chains that hold the polymers together 200 times faster than before, without chemicals and at room temperature. The dissolved polymers can be processed into new adhesives and dyes. The team published their findings in ACS - Applied Materials and Surfaces.
Superabsorbents liquefy under UV light when they have absorbed enough water. They can then be reused.
Ken Pekarsky, KIT
Superabsorbents are found not only in diapers, but in numerous other hygiene and medical products, such as sanitary napkins and bandages. Until now, anyone who wanted to reuse their chemical core had to use strong acids, because the absorbent material is made of sodium polyacrylate. These cross-linked polymers are insoluble in water and cannot be melted down even at high temperatures - they only decompose. However, the acids were able to "cut" the chains that make the polymers stable at 80 degrees Celsius and after about 16 hours, making recycling possible. However, the process is complex and expensive, so reuse of superabsorbents is rare. Every year, around two million tons of them end up in the trash or are incinerated.
Liquid in five minutes instead of 16 hours
Researchers from the Institute of Biological and Chemical Systems, the Institute of Biological Interfaces, and the Institute of Technical Chemistry and Polymer Chemistry at KIT have now discovered that the crosslinked polymers of sodium polyacrylate dissolve after absorbing water under UV light. "The chains connecting the polymers are refracted by light and are then so loose that they float in water and become liquid fibers," explains Pavel Levkin, professor at the Institute of Biological and Chemical Systems. To do this, the researchers cut the absorbent pad out of conventional diapers, moistened it with water and then exposed it to light with a 1,000-watt lamp. After just five minutes, the solid material turned into a liquid that dripped into a collection container. "So this process is about 200 times faster with UV light than with acids," Levkin says.
The recycled polymers have many uses
The team then further processed the raw liquid chemical into new adhesives and dyes using familiar methods. "It was important to observe that the substance is soluble and processable. You can certainly make a lot more from this," the scientist explains.
Clean diapers were used for the tests. In practice, however, it is possible to filter superabsorbents out of soiled materials. "Therefore, nothing stands in the way of a realistic application," Levkin says. In addition, he said, the recycling process could be optimized with solar power in a cost-neutral and ecological way: "We have found a forward-looking strategy for reusing superabsorbers. This could significantly reduce environmental pollution and contribute to a more sustainable use of polymers."
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.




























































