New twist on old chemical process could boost energy efficiency
Advertisement
Chemical reactions on the surface of metal oxides, such as titanium dioxide and zinc oxide, are important for applications such as solar cells that convert the sun's energy to electricity. Now University of Washington scientists have found that a previously unappreciated aspect of those reactions could be key in developing more efficient energy systems.
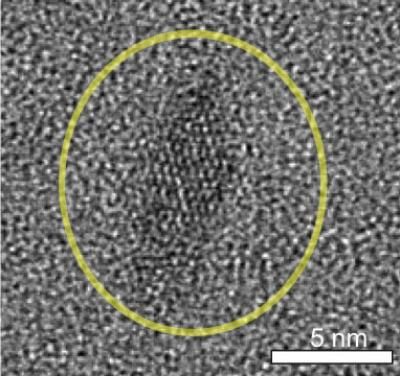
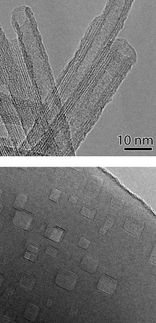
This image from an electron microscope shows a coated titanium dioxide nanocrystal.
Joel Schrauben/James Mayer/UW
Such systems could include, for example, solar cells that would produce more electricity from the sun's rays, or hydrogen fuel cells efficient enough for use in automobiles, said James Mayer, a UW chemistry professor.
"As we think about building a better energy future, we have to develop more efficient ways to convert chemical energy into electrical energy and vice versa," said Mayer, the corresponding author of a paper about the discovery in Science.
Chemical reactions that change the oxidation state of molecules on the surface of metal oxides historically have been seen as a transfer solely of electrons. The new research shows that, at least in some reactions, the transfer process includes coupled electrons and protons.
"Research and manufacturing have grown up around models in which electrons moved but not atoms," Mayer said. The new paper proposes a different model for certain kinds of processes, a perspective that could lead to new avenues of investigation, he said. "In principle this is a path toward more efficient energy utilization."
Coupling the transfer of electrons with the transfer of protons could help reduce the energy barriers to chemical reactions important in many technologies. For example, using solar energy to make fuels such as hydrogen requires that electrons and protons be coupled.
The new perspective also could be important for photocatalytic chemical processes, including those designed for wastewater remediation or to create self-cleaning surfaces, such as the outside of buildings in areas with heavy industrial air pollution.
The research focused specifically on nanoparticles, measured in billionths of a meter, of titanium dioxide and zinc oxide. Titanium dioxide is the most common white pigment, used in paints, coatings, plastics, sunscreen and other materials. Zinc oxide also is used in pigments, coatings and sunscreens, as well as white athletic tape, and also is used in the manufacture of rubber, concrete and other materials. Nanocrystals were used to closely examine chemical processes at the material's surface.
Mayer said the goal of the work is to get those working in various technological areas involving metal oxides to think in different ways about how those technologies work and how to make them more efficient.
The work also could prove important in finding more efficient ways to fuel vehicles of the future, he said. Fuel cells, for example, transform atmospheric oxygen into water by adding both electrons and protons. Coupling those added electrons and protons could make fuel cells more efficient and allow replacement of costly materials such as platinum.
"Chemical fuels are very useful, and they're not going away," Mayer said. "But how do we utilize them better in a non-fossil-fuel world?"