Sustainable iron catalyst for clean hydrogenations
New research reports a clean, sustainable method for performing hydrogenation reactions in water using polymer-supported iron nanoparticles.
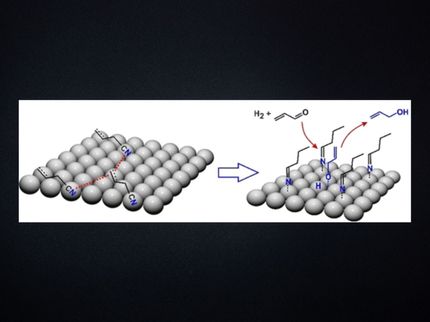
Hydrogenation reactions have numerous industrial applications, including in the petrochemical and pharmaceutical industries, but they usually rely heavily on expensive and rare precious-metal catalysts, which are also toxic. Iron is an abundant and less toxic alternative for catalysing hydrogenations, but its use in industry is limited by rusting in the presence of oxygen and water.
In a joint project, scientists in Canada and Japan have developed amphiphilic polymers that protect iron nanoparticles from being deactivated by water, whilst still allowing the reactants to reach the catalyst active sites. Using this clean, green catalyst, they were able to perform hydrogenations of alkenes, alkynes, aromatic imines and aldehydes that were almost quantitative in most cases.
The researchers also showed that the catalyst can be used in a flow system with little leaching, allowing for continuous hydrogenation at a multi-gram scale and demonstrating its potential application in industrial-scale reactions.
Original publication
R Hudson et al, Green Chem., 2013
Most read news
Original publication
R Hudson et al, Green Chem., 2013
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.