Critical raw materials from electrolysers back into the cycle
Researchers succeed in recycling functional materials for hydrogen production
Advertisement
hydrogen plays a central role in the energy transition. The gas is mainly produced with the help of electrolysers. This process requires critical raw materials such as platinum group metals, rare earths or nickel as catalysts. Researchers at the Helmholtz Institute Freiberg for Resource Technology (HIF), an institute of the Helmholtz-Zentrum Dresden-Rossendorf (HZDR), have now been able to recover these functional materials using innovative flotation processes and liquid-liquid particle separation, thus returning them to the material cycle. The research is part of the H2Giga lead project of the German Federal Ministry of Education and Research (BMBF), which is investigating the longevity and recyclability of hydrogen electrolysers.
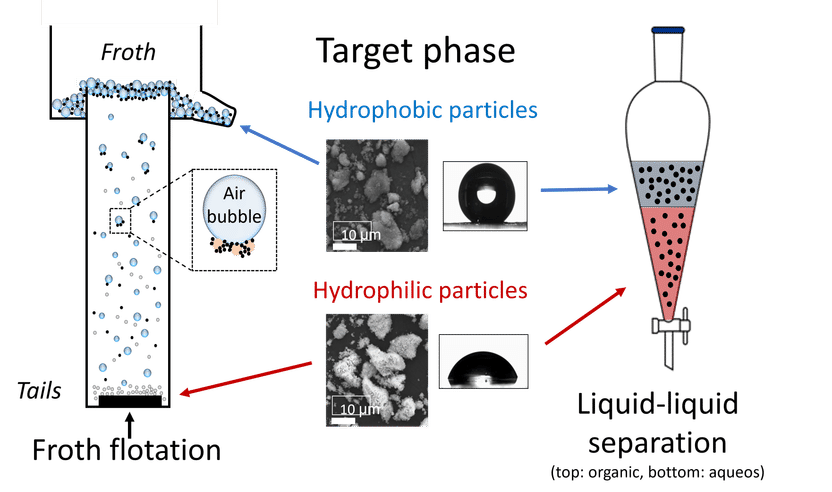
Schematic illustration of the separation methods
Sohyun Ahn
Hydrogen is considered a clean energy source that can help reduce CO2 emissions. The focus here is particularly on green hydrogen, which is produced through the electrolysis of water using renewable energies such as wind and solar power. Hydrogen is used in industry, for example as a raw material for the production of chemicals and steel, as well as in the transport sector, where it is used as a fuel for fuel cell vehicles. Hydrogen can also be used to store surplus energy from renewable sources, making it an important building block for a sustainable and climate-friendly energy future. According to the national hydrogen strategy, Germany is expected to need 95 to 135 terawatt hours of hydrogen in 2030.
Various processes can be used to produce hydrogen - one is water electrolysis: water is broken down into hydrogen and oxygen using an electric current. The catalysts in the electrolyser consist of critical metals, the so-called functional materials. Proton exchange membrane electrolysers (PEM) mainly use metals from the platinum group, such as platinum, iridium and palladium. High-temperature electrolysers use rare earths and nickel. These critical raw materials need to be secured. This is a project that HIF researchers are working on under the leadership of the TU Bergakademie Freiberg in the ReNaRe project.
ReNaRe stands for Recycling - Sustainable Resource Utilization and is part of the H2Giga flagship project. To implement the national hydrogen strategy, the BMBF has set up three flagship projects for Germany's entry into the hydrogen economy. One of these is H2Giga, which focuses on the series production of hydrogen electrolysers. ReNaRe concentrates on the end of life of electrolysers in order to return the materials used, and in particular the critical metals, to the material cycle.
“We are involved in the recycling of PEM and high-temperature electrolysers, as they are easy to dismantle. We use ultra-fine particle separation techniques to recover the functional materials. This is because the critical anode and cathode materials are present as fine particles. Their size corresponds to approximately one hundredth of a human hair. Liquid-liquid particle separation and agglomeration flotation have proven to be suitable for separating the functional materials. The extraction of ultrafine particles uses a sustainable solvent-water circulation system for the effective separation of hydrophobic, i.e. water-repellent cathode catalysts and hydrophilic (water-attracting) anode catalysts. The complementary agglomeration flotation uses an innovative, sustainable hydrophobic binder to enable agglomeration of the particles into a uniform mass. The binder is based on a special emulsion technology, i.e. an oil-water mixture with a very high water content, which selectively agglomerates hydrophobic ultrafine particles. This enables the separation of hydrophilic ultrafine particles by adhesion to gas bubbles and discharge in the foam,” says Sohyun Ahn, PhD student at the HIF, describing the procedure. “With both processes, we were able to recover up to 90 percent of the critical functional materials and return them to the material cycle. An important step towards operating hydrogen electrolysis economically and sustainably.”
In the project, the researchers are now developing a suitable process scheme that enables recycling on a technical scale and is adaptable to current and future technological developments. In addition, technology assessments in the form of life cycle analyses and techno-economic analyses are being carried out in order to quantify the benefits of recycling for sustainability and cost efficiency.
Original publication
Sohyun Ahn, Suvarna Patil, Martin Rudolph; "Ultrafine Particle Recycling─Efficiency of the Hydrophobic Double Emulsion Technique for the Selective Agglomeration and Froth Flotation of Ultrafine Cathode Catalyst Particles from PEM Water Electrolyzers"; ACS Engineering Au, Volume 5, 2024-12-20
Sohyun Ahn, Suvarna Patil, Martin Rudolph; "Influence of surfactants on selective mechanical separation of fine active materials used in high temperature electrolyzers contributing to circular economy"; Industrial Chemistry & Materials, Volume 2, 2024
Sohyun Ahn, Martin Rudolph; "Development of Fine Particle Mechanical Separation Processes with Representative Catalyst Materials for Recycling PEM Water Electrolyzers Exploiting their Wetting Characteristics"; ChemCatChem, Volume 16, 2023-12-15
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Extraction
Extraction is a fundamental process in the chemical laboratory that enables specific components to be isolated and concentrated from a mixture. Whether it's extracting active ingredients from natural products, removing impurities from synthesis products, or preparing analytical samples, extraction is a key step in achieving precise and efficient results in chemical research and analysis.

Topic world Extraction
Extraction is a fundamental process in the chemical laboratory that enables specific components to be isolated and concentrated from a mixture. Whether it's extracting active ingredients from natural products, removing impurities from synthesis products, or preparing analytical samples, extraction is a key step in achieving precise and efficient results in chemical research and analysis.