Fun with Lego (molecules)
Advertisement
A great childhood pleasure is playing with Legos® and marveling at the variety of structures you can create from a small number of basic elements. Such control and variety of superstructures is a goal of polymer chemists, but it is hard to regulate their specific size and how the pieces fit together. Researchers report a simple system to make different nano-architectures with precision.
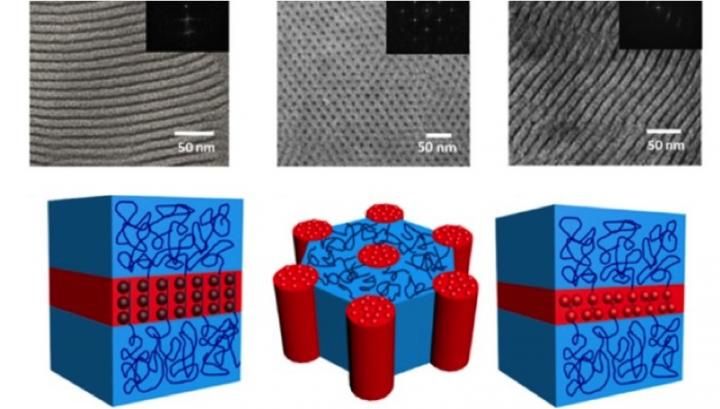
Depending on the relative amounts of different building-block molecules, it is possible to create different sandwich and wheel topologies (shown above in micrographs and below as models).
American Chemical Society. Copyright 2016
Using a variety of highly efficient chemical transformations and other techniques to ensure high yields and purity, Stephen Z. D. Cheng, Yiwen Li, Wen-Bin Zhang and coworkers designed systems to create giant molecules with 'orthogonal' ends, meaning that they only fit together with a specific partner just like Legos®. Depending on the relative amounts of different building-block molecules, these molecules come together in different superstructures -- ranging from cubes to wheels and sandwiches. Eventually, they could be employed in device-creation, where it is crucial to have precise control over the positions of the components.
Original publication
Wei Zhang, Mingjun Huang, Hao Su, Siyu Zhang, Kan Yue, Xue-Hui Dong, Xiaopeng Li, Hao Liu, Shuo Zhang, Chrys Wesdemiotis, Bernard Lotz, Wen-Bin Zhang, Yiwen Li, and Stephen Z. D. Cheng; "Toward Controlled Hierarchical Heterogeneities in Giant Molecules with Precisely Arranged Nano Building Blocks"; ACS Central Science; 2016
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.