Nanotechnology enables new insights into chemical reactions
Researchers have developed a novel method which may speed up the search for optimal catalytic processes
Advertisement
Eighty percent of all products in the chemical industry are manufactured using catalytic processes. catalysis is also indispensable in energy conversion and storage and the treatment of exhaust gases. Reactions between substances are triggered or accelerated by an additional substance, the catalyst. It is important for these processes to run as quickly and efficiently as possible; that protects the environment while also saving time and conserving resources. For this reason, the industry places great importance on optimising catalytic processes. “To do that, you need a deeper understanding of what is going on at the molecular level,” says Jeroen van Bokhoven, Professor of Heterogeneous Catalysis at ETH Zurich and Head of the Laboratory for Catalysis and Sustainable Chemistry at the PSI.
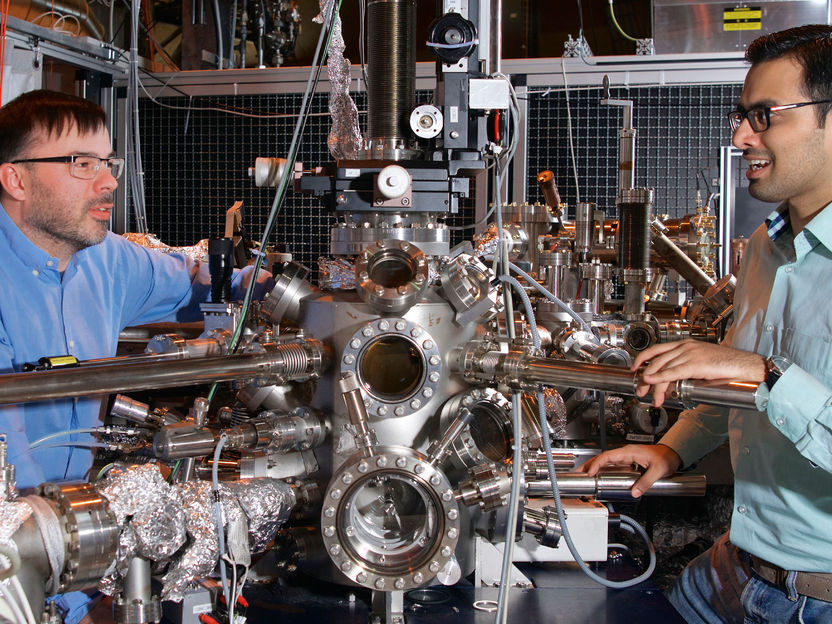
Large-scale research facility that allows to track the chemical reactions of individual nanoparticles: Swiss Light Source SLS of the PSI.
Paul Scherrer Institut/Markus Fischer
Model experiment with unprecedented precision
This deeper understanding can be gained through the new approach that researchers describe in the latest issue of Nature: A team led by van Bokhoven and Waiz Karim built a model system that enables them to study catalysis in the minutest detail. For the model experiment the team converted iron oxide into iron through the addition of hydrogen and with assistance from the catalyst platinum. The platinum splits the molecular hydrogen into elemental hydrogen, which can more easily react with iron oxide to create iron and water.
The main attraction of their model: With state-of-the-art electron-beam lithography, which is normally used in semiconductor technology, the researchers were able to place miniscule particles of just a few atoms each onto a substrate. The researchers positioned these particles in pairs on a grid-like model at different distances from each other. “Thus we were able to test 16 different situations at once and control the size and spacing of the particles with one-nanometre accuracy,” explains Karim, who is affiliated with both ETH Zurich and the PSI.
The precision of the particle positioning is not the only innovation: chemical reactions were also observed with unprecedented accuracy. This was made possible by a special method that uses X-ray microscopy to examine these tiny particles.
Advancing chemical science
As it turned out, though, some chemical phenomena take place on an even smaller scale. One of these is known as the hydrogen spillover effect, which contributes decisively to the efficiency of catalysis with hydrogen. It was discovered in 1964 but had never been understood or visualised in detail. The PSI and ETH researchers succeeded in analysing the effect for the first time with the precision needed, thanks to their new model. As a result, they were able to clarify the circumstances under which it actually occurs.
“Our method rests on three pillars,” says van Bokhoven. “The nanofabrication of the model system, the precise measurement of the chemical reactions, and also the theoretical modelling: In accordance with the experiments we were able to describe the process down to the molecular level.” This, he says, could enable enormous advances in chemical science overall: “We are opening up a whole new dimension for the investigation and understanding of catalytic processes. And with this understanding, industrial production processes can be optimised in a much more targeted way.”