Understanding breakups
Advertisement
As interest and demand for nanotechnology continues to rise, so will the need for nanoscale printing and spraying, which relies on depositing tiny drops of liquid onto a surface. Now researchers from Tsinghua University in Beijing have developed a new theory that describes how such a nanosized droplet deforms and breaks up when it strikes a surface.
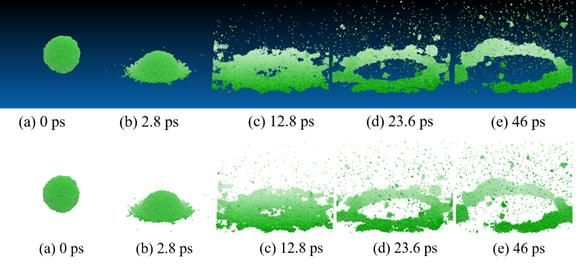
These figures show how a nanodroplet breaks up when it impinges on the solid wall through molecular dynamic simulation in computer. There are 12,195 water molecules represented by the green particles in this figure (the droplet originally has a diameter of 8.6 nm).
Li, Li and Chen
The model could help researchers improve the quality of nanoscale printing and coating, important to everything from printing and coating tiny devices and structures to 3-D printing machines and robots.
When it comes to spraying coatings, for example, the smaller and faster the droplets are when they hit the surface, the better the quality of the coating, said Min Chen, a professor in the Engineering Mechanics Department at Tsinghua University. However, at certain impingement speeds, the droplets will break up and splatter, ruining the coating.
So to improve printing and spraying techniques, we need to better understand the conditions that cause droplets to deform when they hit a surface, as well as how they break. But because experimenting with nanosized droplets is very difficult, researchers often rely on computer simulations.
Bu-Xuan Li and Xin-Hao Li, along with Chen, used a technique called molecular dynamics simulation, in which they simulated every molecule that makes up a droplet of water. Each droplet, consisting of about 12,000 molecules, is about 8.6 nanometers in diameter and hits the surface at speeds of a few hundred meters per second. The computer simulates what happens when the collection of water molecules hits a flat surface.
"We developed an analytical model to describe the deformation process and another to describe the breakup process," Chen said. The deformation model improves upon the team's previous work, "but the breakup model is totally new."
The breakup model combines theory with the results from the simulations, providing a formula that researchers can use to calculate when a droplet will breakup. According to Chen, the model is ready for use in applications.
One limitation is that the model is only verified to work for droplets at the nanoscale, and not for bigger droplets. "The reason is that the way a droplet breaks up is different in macro and nanoscale," Bu-Xuan Li said.
The model also only applies to so-called Newtonian fluids like water. The researchers are now working on developing a model for non-Newtonian fluids, such as crude oil or the gooey mixture of cornstarch and water sometimes known as Oobleck. For example, a non-Newtonian model would be needed for 3-D printing polymers and biomaterials, such as human tissue and organs.
The model is also applicable for describing how water droplets collide with aircraft and form ice, which is a safety hazard. These water droplets, suspended in clouds, typically range from 20 to 50 micrometers -- bigger than those in the simulations. Still, Chen said, their model is useful because not much is known about how those water droplets impinge on aircraft.