The layered look of lithium sulfur
Advertisement
In the quest for longer-lasting lithium rechargeable batteries, some researchers are looking to use sulfur as electrodes. The chemistry of lithium-sulfur, in theory, could allow electric vehicles to go twice as far as conventional batteries. But, in practice, unwanted chemical reactions clog the electrodes quickly, meaning a short lifespan for the batteries as their ability to hold a charge fades.
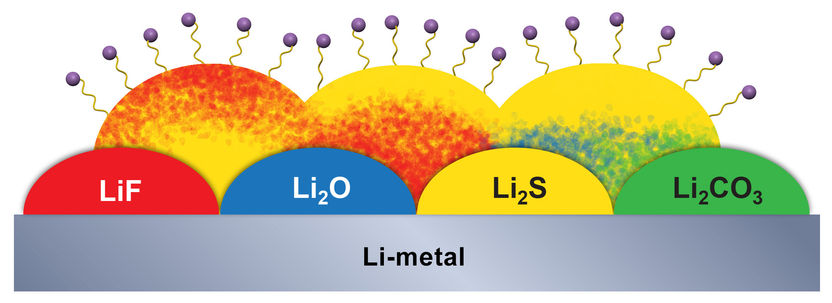
In a battery in which one electrode contains lithium and the other sulfur, unwanted chemical byproducts build up on the lithium electrode (gray). They form three layers with distinct origins, here represented as solid colors, mixed colors, and wiggly lines and balls.
PNNL
Now, researchers have gotten the best look yet at what's going on when a lithium-sulfur battery is charging and discharging. First they had to adapt a laboratory instrument to trap the products of the unwanted chemical reactions. But then the team saw how the components of the batteries — electrodes and the liquid electrolytes that help create the electric current — interact and form an interfering layer on the electrodes.
Understanding how the layer builds up might help scientists solve the lithium-sulfur fading problem, which could lead to more affordable batteries. "Sulfur is significantly cheaper than current cathode materials in lithium-ion batteries," said researcher Vijay Murugesan of the Department of Energy's Pacific Northwest National Laboratory . "So the total cost of a lithium-sulfur battery will be low."
Original publication
Manjula I. Nandasiri, Luis E. Camacho-Forero, Ashleigh M. Schwarz, Vaithiyalingam Shutthanandan, Suntharampillai Thevuthasan, Perla B. Balbuena, Karl T. Mueller, and Vijayakumar Murugesan; "In Situ Chemical Imaging of Solid-Electrolyte Interphase Layer Evolution in Li–S Batteries"; Chemistry of Materials; 2017
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.































































