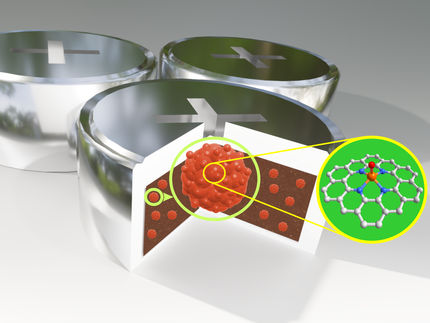
The inclusion of small molecules changes the distance between layers in lamellar material
Advertisement
A crystal has a defined structure that cannot be altered much once it is formed-or such was the belief until now. Spanish chemists have recently demonstrated this assertion to be false; they have produced a layered material whose porosity can be changed from one moment to the next. This could signal the birth of a new type of material with tailored pores that respond to external signals. Microporous materials can take up "guest" molecules, and are highly coveted for selective catalysts, ion exchangers, or storage for pharmaceuticals or other chemical agents.
The team, led by Ernesto Brunet, selected gamma-zirconium phosphate, which is known to have a layered structure, as their starting material. The surfaces of the individual layers have phosphate groups [PO4] sticking out of them, and these can easily be replaced without changing the structure of the layers. The researchers replaced some of the phosphates with short polyethylene glycol chains that had a phosphonic acid group [PO(OH)2] at each end. These "diphosphonates" forced out one phosphate on each of two adjacent layers, bridging the layers together. The chains support the space between the layers like columns in an arcade. The researchers then exchange the remaining phosphate groups for hypophosphite groups [H2PO2]. These are embedded in the surface of the layer so that their apolar PH2 side protrudes into the space between the layers. In contrast to the product of the precursor step, the apolar interactions between the layers now cause the columns to lie flat (parallel to the layers), which significantly decreases the distance between the layers. If this material is then treated with basic methylamine, it abruptly soaks it up-within a narrow pH range-and the separation between layers increases by almost 70%. How? Polar molecules like methylamine initially cannot enter into the apolar interlayer spaces. However, the anchors for the columns, the phosphonate groups, are polar. Once the pH value reaches about 4.5, the attraction between the basic methylamine and the acidic phosphonates is so strong that individual methylamine ions force their way in at the edges of the crystals. These ions are relatively large compared to the distance between the layers. Like a wedge, they drive the rigid layers apart and cause the columns to stand upright. A few wedges are enough to completely neutralize the apolar attractive forces between the layers and bring all of the columns into an upright position. The distance between the layers-and with it the porosity-increases abruptly. "Such a high sensitivity of microcrystalline porosity towards the incorporation of small molecules is thus far unique," says Brunet.
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Breakthrough: Scientists develop artificial molecules that behave like real ones - The uses of this new technique are endless: ‘We have only begun to imagine what we can use this for. We have so many ideas...’