New process for the production of efficient catalyst systems for the synthesis of methanol
Advertisement
Methanol is not only an important starting material for the chemical industry; it is gaining attention as an energy source for fuel cells. In contrast to hydrogen, liquid methanol would be easy to transport and could be distributed by means of the existing network of filling stations. It is no wonder that the search is on for better catalysts for the synthesis of methanol. Researchers working for a Special Research Area of the German Research Society (SFB 558) based at the Ruhr University in Bochum have reported success on this front with an unusual vaporization technique.
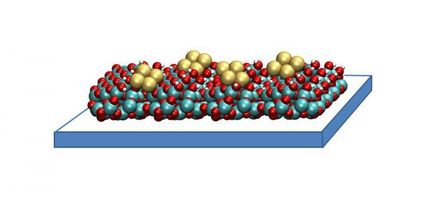
Industrial production of methanol usually involves the conversion of synthesis gas, a mix of carbon dioxide, carbon monoxide, and hydrogen, using copper/zinc oxide catalysts. The effectiveness of the catalysts is clearly affected by interactions between the metallic copper and the zinc oxide, which acts as a support. The team headed by Roland A. Fischer thus looked for a way to maximize contact at the interface between the copper and zinc oxide. This led them to the idea of using porous silicate materials as a support for their catalyst systems. These materials have the advantage of having both a very highly specific surface and a precisely controllable nanoscopic pore structure; they have also previously proven to be excellent support materials in many cases. Instead of applying the catalytically active substances -- copper and zinc oxide -- by means of conventional impregnation processes, the Bochum researchers used a method called organometallic chemical vapor deposition. First they vaporize an oxygen-containing organocopper compound in a vacuum. The vapor is then firmly adsorbed onto the silicate support. Diethyl zinc is then deposited in the same manner and the material is carefully heated. On the molecular level, the following occurs: the zinc atoms take the place of copper atoms, which are deposited as metallic copper. Heating causes all of the organic compounds to be burned off, leaving the zinc behind as zinc oxide. What is special about all of this is that the copper and zinc oxide are extremely finely divided, so that they are in particularly close contact with each other. In this way, the researchers obtained catalytic materials that are at least as effective as the classic copper/zinc oxide catalysts. "The catalytic activity of one sample exceeds that of the classic system to a surprising degree," says Fischer. "The reason for this is the special three-dimensional pore structure of this silicate support, which allows for an especially efficient diffusion of the penetrating vapors."
Other news from the department research and development
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.