To use all functions of this page, please activate cookies in your browser.
my.chemeurope.com
With an accout for my.chemeurope.com you can always see everything at a glance – and you can configure your own website and individual newsletter.
- My watch list
- My saved searches
- My saved topics
- My newsletter
Hammett acidity function
The Hammett acidity function is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. In such solutions, simple approximations such as the Henderson-Hasselbalch equation are no longer valid due to the variations of the activity coefficients in highly concentrated solutions. The Hammett acidity function is used in fields such as physical organic chemistry for the study of acid-catalyzed reactions, because some of these reactions use acids in very high concentrations, or even neat (pure).[1] Product highlightThe Hammett acidity function, H0, is used as a pH surrogate. It is defined as
where a is the activity, and γ are the activity coefficients of a base B and its conjugate acid BH+. H0 can be calculated using an equation analogous to the Henderson-Hasselbalch equation:
where pKBH+ is −log(K) for the dissociation of BH+. By using bases with very negative pKBH+ values, the H0 scale may be extended to negative values. Hammett originally used a series of anilines with electron-withdrawing groups for the bases.[1] On this scale, pure H2SO4 (18.4 M) has a H0 value of −12, and pyrosulfuric acid has H0 ~ −15.[2] Take note that the Hammett acidity function clearly avoids water in its equation. It is a generalization of the pH scale—in a dilute aqueous solution (where B is H2O), pH is very nearly equal to H0. By using a solvent-independent quantitative measure of acidity, the implications of the leveling effect are eliminated, and it becomes possible to directly compare the acidities of different substances (e.g. using pKa, HF is weaker than HCl in water but stronger than HCl in glacial acetic acid; however, pure HF is "stronger" than HCl because the H0 of pure HF is higher than that of pure HCl.)[citation needed] H0 for some concentrated acids:[citation needed]
For mixtures (e.g., partly diluted acids in water), the acidity function depends on the composition of the mixture and has to be determined empirically. Graphs of H0 vs mole fraction can be found in the literature for many acids.[1] Although the Hammett acidity function is the best known acidity function, other acidity functions have been developed by authors such as Arnett, Cox, Katrizky, Yates, and Stevens.[1] References
Categories: Acid-base chemistry | Physical organic chemistry | Superacids |
| This article is licensed under the GNU Free Documentation License. It uses material from the Wikipedia article "Hammett_acidity_function". A list of authors is available in Wikipedia. |





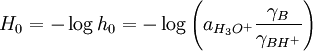
![H_{0} = \mbox{p}K_{BH^+} + \log \frac{[B]}{[BH^+]}](images/math/2/e/1/2e1ce7ec35aec0eb867a815ff9fa72f9.png)


