New material captures carbon dioxide
The captured CO2 can be converted into useful organic materials
Advertisement
A new material that can selectively capture carbon dioxide (CO2) molecules and efficiently convert them into useful organic materials has been developed by researchers at Kyoto University, along with colleagues at the University of Tokyo and Jiangsu Normal University in China. They describe the material in the journal Nature Communications.
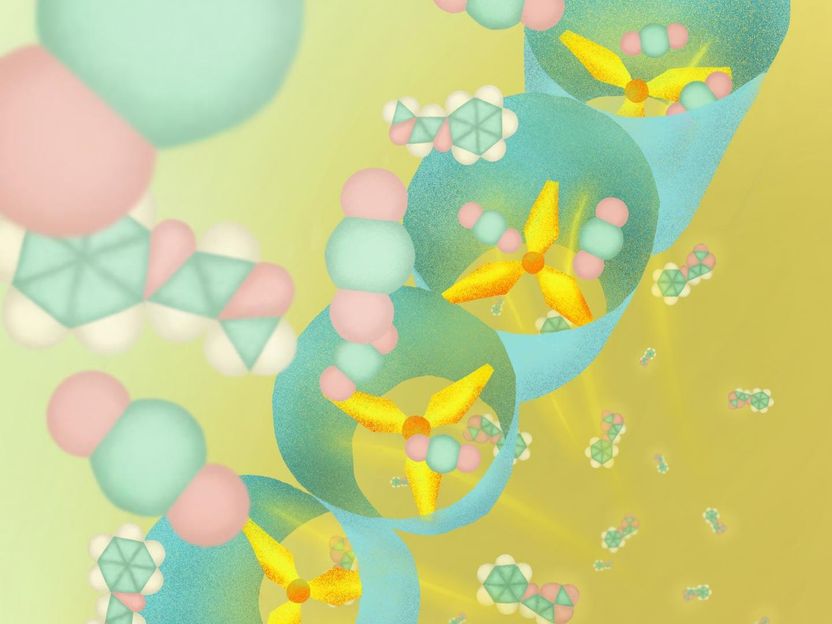
This new porous coordination polymer has propeller-shaped molecular structures that enables selectively capturing CO2, and efficiently convert the CO2 into useful carbon materials.
Illustration by Mindy Takamiya (CC BY 4.0: https://creativecommons.org/licenses/by/4.0/)
Human consumption of fossil fuels has resulted in rising global CO2 emissions, leading to serious problems associated with global warming and climate change. One possible way to counteract this is to capture and sequester carbon from the atmosphere, but current methods are highly energy intensive. The low reactivity of CO2 makes it difficult to capture and convert it efficiently.
"We have successfully designed a porous material which has a high affinity towards CO2 molecules and can quickly and effectively convert it into useful organic materials," says Ken-ichi Otake, Kyoto University materials chemist from the Institute for Integrated Cell-Material Sciences (iCeMS).
The material is a porous coordination polymer (PCP, also known as MOF; metal-organic framework), a framework consisting of zinc metal ions. The researchers tested their material using X-ray structural analysis and found that it can selectively capture only CO2 molecules with ten times more efficiency than other PCPs.
The material has an organic component with a propeller-like molecular structure, and as CO2 molecules approach the structure, they rotate and rearrange to permit C02 trapping, resulting in slight changes to the molecular channels within the PCP - this allows it to act as molecular sieve that can recognize molecules by size and shape. The PCP is also recyclable; the efficiency of the catalyst did not decrease even after 10 reaction cycles.
"One of the greenest approaches to carbon capture is to recycle the carbon dioxide into high-value chemicals, such as cyclic carbonates which can be used in petrochemicals and pharmaceuticals," says Susumu Kitagawa, materials chemist at Kyoto University.
After capturing the carbon, the converted material can be used to make polyurethane, a material with a wide variety of applications including clothing, domestic appliances and packaging.
This work highlights the potential of porous coordination polymers for trapping carbon dioxide and converting into useful materials, opening up an avenue for future research into carbon capture materials.

































































