Researchers develop a membrane that stabilises lithium batteries
"Lithium is just like an unruly child”
Advertisement
For many people, battery-powered electronic devices like cell phones, laptops, and even electric vehicles, are now a necessity. However, these devices typically require charging at least once a day. Increasing the length of time between charges requires the development of batteries that can store more energy. While lithium metal electrodes promise to significantly improve energy density, their lack of stability means the batteries have a short service life and are associated with serious safety hazards.
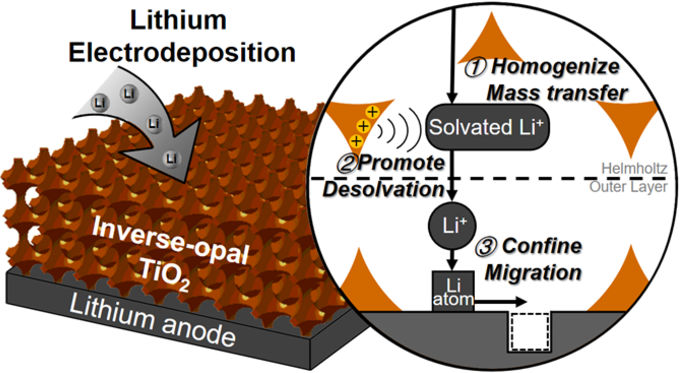
How the inverse-opal structured TIO2 films work
GUORAN LI
In a study published in the KeAi journal Green Energy & Environment, a group of researchers from Nankai University in China and the UK’s University of Cambridge, demonstrate a new method they have developed to stabilise lithium electrodes. It draws on an inverse-opal structured electrode interface protective film, that effectively controls the ion electrodeposition process on the electrode surface.
"Lithium is just like an unruly child,” explains one of the study's authors, Guoran Li, a professor in Materials Chemistry at Nankai University's School of Materials Science and Engineering. “Its capricious behaviour in the electrode reaction process leads to an uneven electrode surface and sharp dendrites that can pierce the separator and cause a fire. These make the battery unstable and unsafe.”
To control that unruliness, Prof. Li and his fellow researchers decided to move away from the traditional method used to protect the electrode surfaces from corrosion, and develop a membrane with a regular structure and active components to manage the behaviour of lithium.
He says: “The highly ordered channels in the inverse-opal structure even out the lithium ion distribution, and effectively regulate every stage of the electrodeposition process to achieve the final target, i.e., make the lithium metal electrode work stably for the batteries.”
According to Wu Xuewen, the PhD student who led to the investigation and data curation, this is a valuable research result in the field of lithium (Li) metal electrodes. "Our research shows that the regular structure and active components of the protective membrane can effectively regulate the electrode reaction process to improve the final electrochemical performance." He adds: “We hope that our study can provide a new perspective for the detailed exploration of the reaction process of Li metal electrodes, and promote the practical application of high-performance Li metal batteries."
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.